The following was written by Dr. Raj Karnatak, 2nd year ID fellow at UNMC; a reflection of his current Antimicrobial Stewardship/Infection Control rotation:
The UNMC Infectious Diseases fellowship antimicrobial stewardship and infection control rotation provides robust training for fellows in both antimicrobial stewardship and infection control. Training is well designed with education in all the core elements of stewardship and infection control. Fellows attend dedicated lectures from the experts in the field of infection control and antimicrobial stewardship. Throughout the rotation fellows actively participate in hospital acquired infections (HAI) surveillance and infection prevention, and antimicrobial stewardship interventions. In the Infection control training, we learn about the role of healthcare epidemiology, surveillance and prevention of healthcare-associated infections including C. difficile, central-line associated bloodstream infections, multidrug-resistant organisms, ventilator-associated events, and surgical site infections.
 Our antimicrobial stewardship training is concentrated on learning the CDC core elements of antimicrobial stewardship and implementing principles of antimicrobial stewardship in healthcare settings (Inpatient, outpatient, long-term care facility). Fellows actively engage in quality improvement in the infection control and antimicrobial stewardship and also work closely with our Stewardship Pharmacy Coordinator. Learning stewardship core elements present you to principles that can very well be applied to a wide variety of QI efforts. As fellows we are fully integrated into the stewardship team during our rotation, and besides attending key meetings where brainstorming stewardship issues occur and decisions are made, we actively participate in daily telephone audit-and-feedback. This gives us needed practice with communicating with prescribers, troubleshooting common problems and helps us to be better Infectious Disease Doctors. We are also participating in the IDSA Antimicrobial Stewardship Curriculum pilot. In this formal training, our curriculum Directors Drs. Van Schooneveld and Marcelin meet with us regularly for case-studies, role playing and module reviews, where we discuss approaches to handling difficult situations as #Stewies.
Our antimicrobial stewardship training is concentrated on learning the CDC core elements of antimicrobial stewardship and implementing principles of antimicrobial stewardship in healthcare settings (Inpatient, outpatient, long-term care facility). Fellows actively engage in quality improvement in the infection control and antimicrobial stewardship and also work closely with our Stewardship Pharmacy Coordinator. Learning stewardship core elements present you to principles that can very well be applied to a wide variety of QI efforts. As fellows we are fully integrated into the stewardship team during our rotation, and besides attending key meetings where brainstorming stewardship issues occur and decisions are made, we actively participate in daily telephone audit-and-feedback. This gives us needed practice with communicating with prescribers, troubleshooting common problems and helps us to be better Infectious Disease Doctors. We are also participating in the IDSA Antimicrobial Stewardship Curriculum pilot. In this formal training, our curriculum Directors Drs. Van Schooneveld and Marcelin meet with us regularly for case-studies, role playing and module reviews, where we discuss approaches to handling difficult situations as #Stewies.
 As a part of my stewardship project, I am working on developing an institutional guidance document for antibiotic management of acute rhinosinusitis and pharyngitis in the outpatient setting. My other project is in infection control for the prevention of ventilator-associated events. I also had the opportunity to work with a larger multidisciplinary sepsis group for the development and implementation of institutional sepsis protocol. As a budding Infectious Diseases physician with particular interest in Critical Care Medicine, I know that Antimicrobial Stewardship is essential to any job I take post-fellowship, and I am thrilled to be at an institution that values it so highly.
As a part of my stewardship project, I am working on developing an institutional guidance document for antibiotic management of acute rhinosinusitis and pharyngitis in the outpatient setting. My other project is in infection control for the prevention of ventilator-associated events. I also had the opportunity to work with a larger multidisciplinary sepsis group for the development and implementation of institutional sepsis protocol. As a budding Infectious Diseases physician with particular interest in Critical Care Medicine, I know that Antimicrobial Stewardship is essential to any job I take post-fellowship, and I am thrilled to be at an institution that values it so highly.
 The highlights of Nebraska ASAP initiative include:
The highlights of Nebraska ASAP initiative include: • Organized the inaugural “Antimicrobial Stewardship Summit” for the state of Nebraska on June 1st 2018 to provide education to ASP program leaders (over 250 healthcare workers attended the summit)
• Organized the inaugural “Antimicrobial Stewardship Summit” for the state of Nebraska on June 1st 2018 to provide education to ASP program leaders (over 250 healthcare workers attended the summit)
 We are also focusing efforts on Outpatient Antimicrobial Stewardship. While this program is still in its nascent stages, in collaboration with
We are also focusing efforts on Outpatient Antimicrobial Stewardship. While this program is still in its nascent stages, in collaboration with 

 The dogs had a moderate interrater reliability with a Cohen’s kappa of 0.52. Both dogs had about 85% specificity of toxigenic C. difficile detection but the German Shepherd’s sensitivity of detection out-sniffed the Border Collie Pointer (92% vs 78% respectively). Positive predictive value for both dogs was <50% and negative predictive value was >95% for both dogs. Interrater variability necessitates individualized dog training; and it is curious that two different species were used – could there be a genetic predisposition for “better” olfactory receptors in certain species?
The dogs had a moderate interrater reliability with a Cohen’s kappa of 0.52. Both dogs had about 85% specificity of toxigenic C. difficile detection but the German Shepherd’s sensitivity of detection out-sniffed the Border Collie Pointer (92% vs 78% respectively). Positive predictive value for both dogs was <50% and negative predictive value was >95% for both dogs. Interrater variability necessitates individualized dog training; and it is curious that two different species were used – could there be a genetic predisposition for “better” olfactory receptors in certain species?
 If two different canine species could not agree on whether or not the stool smelled “diffy”, where does that leave humans, whose olfactory capabilities are thought to be
If two different canine species could not agree on whether or not the stool smelled “diffy”, where does that leave humans, whose olfactory capabilities are thought to be 
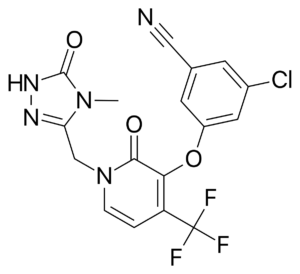 Doravirine (DOR) is a novel NNRTI that provides a similar efficacy for the treatment of HIV infection with activity against HIV variants that are resistant to efavirenz (EFV) and rilpivirine (RPV). Doravirine offers a better safety profile without neuropsychiatric adverse effects, minimal drug-drug interactions and is unaffected by food intake and need for an acidic absorption environment. In August, 2018, doravirine was approved by the FDA for use and will be available solely (Pifeltro™) or as a single tablet regimen (Delstrigo™) in combination with lamivudine (3TC) and tenofovir disoproxil fumurate (TDF).
Doravirine (DOR) is a novel NNRTI that provides a similar efficacy for the treatment of HIV infection with activity against HIV variants that are resistant to efavirenz (EFV) and rilpivirine (RPV). Doravirine offers a better safety profile without neuropsychiatric adverse effects, minimal drug-drug interactions and is unaffected by food intake and need for an acidic absorption environment. In August, 2018, doravirine was approved by the FDA for use and will be available solely (Pifeltro™) or as a single tablet regimen (Delstrigo™) in combination with lamivudine (3TC) and tenofovir disoproxil fumurate (TDF). DRIVE-AHEAD2: A randomized, double-blind, phase III trial compared doravirine to another NNRTI, efavirenz. Adults with HIV-1 infection naïve to ART, HIV RNA >1,000 copies/ml, and CD4 >100/mm3 were randomized to receive DOR 100mg with 3TC 300mg/TDF 300mg or EFV 600mg with TDF 300mg/emtricitabine (FTC) 200mg. The primary endpoint of the study measured virologic response with the proportion of patients achieving HIV RNA <40 copies/ml at week 48. Comparisons between each arm were similar, 77% in DOR arm vs. 78% in EFV arm, demonstrating non-inferiority. Clinical adverse events deemed drug-related were reported in 31% of patients in DOR arm and 56% in EFV arm. Dizziness (6.5% DOR vs. 25% EFV) and abnormal dreams (5.6% DOR vs. 14.8% EFV) had the largest variation between the two groups. Only one emergent NNRTI mutation arose to the DOR group, K101K/E mutation, which causes intermediate resistance to RPV and low-level resistance to EFV.
DRIVE-AHEAD2: A randomized, double-blind, phase III trial compared doravirine to another NNRTI, efavirenz. Adults with HIV-1 infection naïve to ART, HIV RNA >1,000 copies/ml, and CD4 >100/mm3 were randomized to receive DOR 100mg with 3TC 300mg/TDF 300mg or EFV 600mg with TDF 300mg/emtricitabine (FTC) 200mg. The primary endpoint of the study measured virologic response with the proportion of patients achieving HIV RNA <40 copies/ml at week 48. Comparisons between each arm were similar, 77% in DOR arm vs. 78% in EFV arm, demonstrating non-inferiority. Clinical adverse events deemed drug-related were reported in 31% of patients in DOR arm and 56% in EFV arm. Dizziness (6.5% DOR vs. 25% EFV) and abnormal dreams (5.6% DOR vs. 14.8% EFV) had the largest variation between the two groups. Only one emergent NNRTI mutation arose to the DOR group, K101K/E mutation, which causes intermediate resistance to RPV and low-level resistance to EFV. DRIVE-FORWARD3: A randomized, controlled, double-blind, phase III, non-inferiority trial compared doravirine to ritonavir-boosted darunavir, a protease inhibitor. Adults with HIV-1 infection naïve to ART, with plasma HIV RNA >1,000 copies/ml were screened and randomized to receive DOR 100mg or DRV 800mg/RTV 100mg (DRV/r), in combination with either TDF/FTC or ABC/3TC based on investigator choice. The proportion of patients that achieved plasma HIV-1 RNA <50 copies/ml at week 48 defined the primary endpoint of this trial. Doravirine showed non-inferiority to ritonavir boosted darunavir, with 84% in DOR arm vs. 80% in DRV/r arm achieving success with HIV RNA <50c/ml at week 48. Clinical adverse events due to drug therapy were reported in 31% in DOR and 32% in DRV/r group, with diarrhea comprising 5% of DOR patients vs. 13% of DRV/r patients. Lab abnormalities were similar between the two regimens, except LDL-cholesterol increases in <1% of DOR patients vs. 9% of DRV/r patients. Resistance testing was performed in 15 protocol-defined virologic failure (PDVF) patients, and within this group no emergent mutations to DOR were found. One case of resistance was found in a patient that discontinued treatment because of non-compliance at week 24, thus was not included in the PDVF category, encompassing resistance to DOR (V106I, H221Y, F227C) and FTC (M184V).
DRIVE-FORWARD3: A randomized, controlled, double-blind, phase III, non-inferiority trial compared doravirine to ritonavir-boosted darunavir, a protease inhibitor. Adults with HIV-1 infection naïve to ART, with plasma HIV RNA >1,000 copies/ml were screened and randomized to receive DOR 100mg or DRV 800mg/RTV 100mg (DRV/r), in combination with either TDF/FTC or ABC/3TC based on investigator choice. The proportion of patients that achieved plasma HIV-1 RNA <50 copies/ml at week 48 defined the primary endpoint of this trial. Doravirine showed non-inferiority to ritonavir boosted darunavir, with 84% in DOR arm vs. 80% in DRV/r arm achieving success with HIV RNA <50c/ml at week 48. Clinical adverse events due to drug therapy were reported in 31% in DOR and 32% in DRV/r group, with diarrhea comprising 5% of DOR patients vs. 13% of DRV/r patients. Lab abnormalities were similar between the two regimens, except LDL-cholesterol increases in <1% of DOR patients vs. 9% of DRV/r patients. Resistance testing was performed in 15 protocol-defined virologic failure (PDVF) patients, and within this group no emergent mutations to DOR were found. One case of resistance was found in a patient that discontinued treatment because of non-compliance at week 24, thus was not included in the PDVF category, encompassing resistance to DOR (V106I, H221Y, F227C) and FTC (M184V).


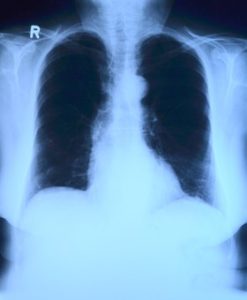 In the second article, “
In the second article, “
Recent Comments