This summer, we have seen several cases of West Nile infection, prompting many questions related to the infection.
So, what’s all the buzz about West Nile? Drs. Jasmine Marcelin and Kelly Cawcutt compiled some answers to Frequently Asked Questions about West Nile.
What is West Nile Virus? West Nile Virus is spread via mosquito bites. Mosquitos get the virus by biting infected birds. In North America, the virus causes disease seasonally from summer through fall, with peak infections occurring in August and September. 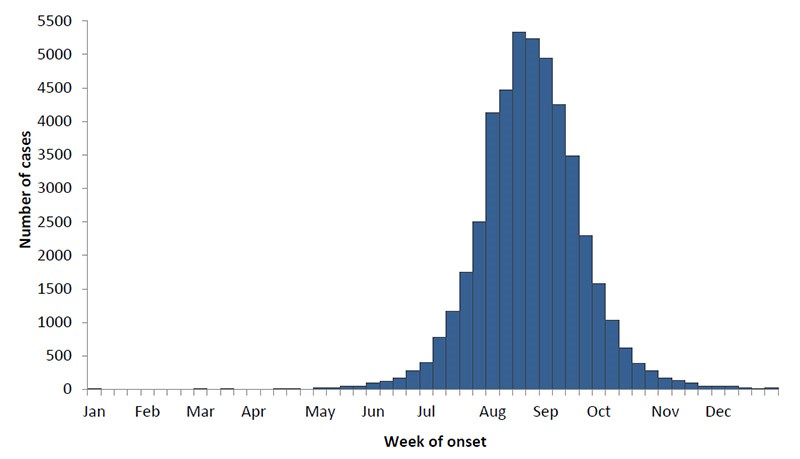
Where can you get it? West Nile cases have been reported in all of the continental US states in the past, and most thus far in 2018. This year, as of CDC’s September 4 report, the leading states with cases of West Nile were North Dakota, Nebraska and South Dakota, respectively.
Who gets it? Anyone can get West Nile Virus. Besides mosquito bites, it has been spread through blood transfusions, organ donation, vertically from mother to fetus and via breast milk. Patients over age 60 are higher risk for developing infection, including serious complications.
What are the symptoms/signs of West Nile Virus infections? Most patients will not develop symptoms of infection, with only about 20% of patients falling ill. Symptomatic infection may include fever, muscle aches, headache, weakness or other nonspecific symptoms of a viral illness. According to the CDC, approximately 1 in 150 patients will develop severe, potentially fatal, infections from West Nile Virus.
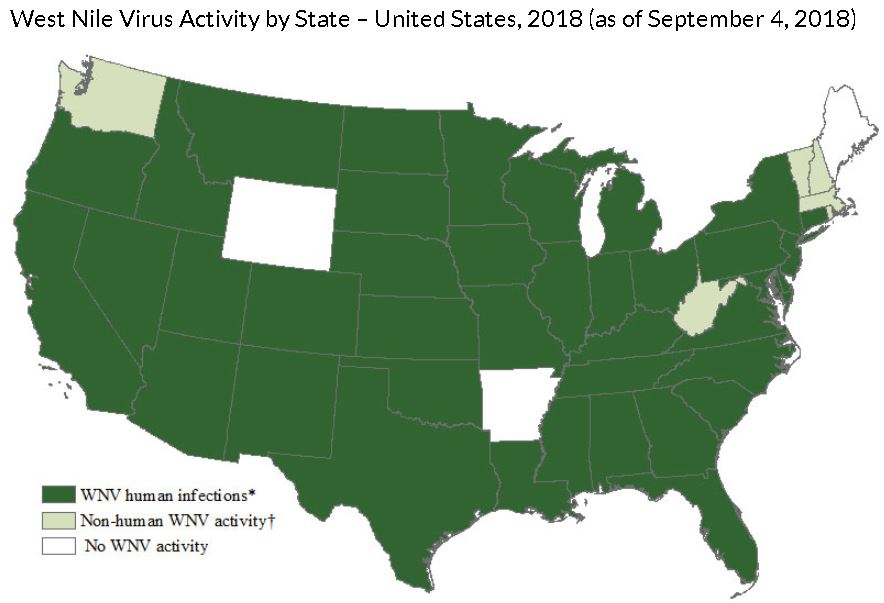
Are we seeing more cases than usual this year? West Nile Virus infections vary year-to-year, but yes, there are more confirmed cases in Douglas County this year compared to last year (7 confirmed cases in 2017). In Nebraska, we already have seen more cases thus far than the entire season last year (79 cases and 4 deaths already compared to 68 cases and 2 deaths last year), but the season is not over. Over the past several years, there are many seasons with much higher rates of infection in the state.
 Is there more neuroinvasive disease than in the past? Neuroinvasive disease (e.g., meningitis, encephalitis, or acute flaccid paralysis) occurred in about 46% of West Nile Virus infections over the past 18 years of surveillance. In the state of Nebraska, 42of the 79 cases of West Nile Virus infections this year have been classified as neuroinvasive. Nationally, of the 231 cases of West Nile Virus infections reported nationally to the CDC to date, 55% have been neuroinvasive cases. However, we are just at the peak of the season and will likely see more cases that are not neuroinvasive as the season progresses.
Is there more neuroinvasive disease than in the past? Neuroinvasive disease (e.g., meningitis, encephalitis, or acute flaccid paralysis) occurred in about 46% of West Nile Virus infections over the past 18 years of surveillance. In the state of Nebraska, 42of the 79 cases of West Nile Virus infections this year have been classified as neuroinvasive. Nationally, of the 231 cases of West Nile Virus infections reported nationally to the CDC to date, 55% have been neuroinvasive cases. However, we are just at the peak of the season and will likely see more cases that are not neuroinvasive as the season progresses.
 How do I test for it? Should I re-test? In general, diagnosis of West Nile Virus requires testing blood for antibodies to the virus. IgM antibodies present indicate a current or recent infection. If a person has neurologic symptoms (concerning for neuroinvasive disease), lumbar puncture is recommended with testing of the cerebrospinal fluid for West Nile IgM. If the initial IgM test is negative but suspicion for West Nile Virus is high, the antibody test should be repeated in 10 days (Convalescent testing), particularly if symptoms persist.
How do I test for it? Should I re-test? In general, diagnosis of West Nile Virus requires testing blood for antibodies to the virus. IgM antibodies present indicate a current or recent infection. If a person has neurologic symptoms (concerning for neuroinvasive disease), lumbar puncture is recommended with testing of the cerebrospinal fluid for West Nile IgM. If the initial IgM test is negative but suspicion for West Nile Virus is high, the antibody test should be repeated in 10 days (Convalescent testing), particularly if symptoms persist.
What is the treatment? The best treatment is time.There is no specific medication to treat infections due to West Nile Virus. Patients with this infection usually require symptomatic management, like fluids, supplemental oxygen, etc. Other treatments like antivirals, intravenous immunoglobulin (IVIG), or plasma exchange have not been proven to be beneficial.
 Can it be prevented? There is no vaccine for West Nile Virus. You can prevent West Nile infection by preventing mosquito bites. Use long sleeves, pants and insect repellents such as DEET or Picardin. Check out the EPA repellent information to help choose the best option here: https://www.epa.gov/insect-repellents/find-repellent-right-you
Can it be prevented? There is no vaccine for West Nile Virus. You can prevent West Nile infection by preventing mosquito bites. Use long sleeves, pants and insect repellents such as DEET or Picardin. Check out the EPA repellent information to help choose the best option here: https://www.epa.gov/insect-repellents/find-repellent-right-you
References:
https://www.cdc.gov/westnile/index.html
https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2018/disease-cases-state-2018.html
 Dr. Angela Hewlett has been elected to the Executive Board of the Musculoskeletal Infection Society and will serve as MSIS President in 2020. She was recently an invited delegate at the International Consensus Meeting on Periprosthetic Joint Infection in Philadelphia, where experts in orthopedic infections from around the world (over 80 countries were represented) came together to develop a document based on the available scientific evidence and consensus when evidence is lacking, that can be used to improve the care of patients with musculoskeletal infections. Dr. Hewlett will be an invited speaker at #IDWeek2018 at the Meet the Professor Session on outbreak preparedness. She also published the book, Bioemergency Planning: A Guide for Healthcare Facilities.
Dr. Angela Hewlett has been elected to the Executive Board of the Musculoskeletal Infection Society and will serve as MSIS President in 2020. She was recently an invited delegate at the International Consensus Meeting on Periprosthetic Joint Infection in Philadelphia, where experts in orthopedic infections from around the world (over 80 countries were represented) came together to develop a document based on the available scientific evidence and consensus when evidence is lacking, that can be used to improve the care of patients with musculoskeletal infections. Dr. Hewlett will be an invited speaker at #IDWeek2018 at the Meet the Professor Session on outbreak preparedness. She also published the book, Bioemergency Planning: A Guide for Healthcare Facilities. Dr. Jasmine Marcelin was awarded the SHEA Race Against Resistance Scholarship, funding provided for a new Antimicrobial Stewardship clincian to learn more about the field. Dr. Marcelin partnered with Physicians Weekly to co-moderate two twitter chats on Minorities in medicine, and was recently featured on the cover of the July issue of Helio Infectious Diseases News in an article discussing Women in Infectious Diseases.
Dr. Jasmine Marcelin was awarded the SHEA Race Against Resistance Scholarship, funding provided for a new Antimicrobial Stewardship clincian to learn more about the field. Dr. Marcelin partnered with Physicians Weekly to co-moderate two twitter chats on Minorities in medicine, and was recently featured on the cover of the July issue of Helio Infectious Diseases News in an article discussing Women in Infectious Diseases. Dr. Susan Swindells was awarded the Department of Medicine Faculty Clinical and Educational Mentoring Award.
Dr. Susan Swindells was awarded the Department of Medicine Faculty Clinical and Educational Mentoring Award. Dr. Trevor Van Schooneveld was recently named UNMC College of Medicine Resident Program Director of the Month in September (ID Fellowship Program Director)
Dr. Trevor Van Schooneveld was recently named UNMC College of Medicine Resident Program Director of the Month in September (ID Fellowship Program Director)
 Dr. Sara Bares was recently inducted into the UNMC Interprofessional Academy of Educators. She also recently was awarded the ACTG Network Minority HIV Investigator Mentoring Award.
Dr. Sara Bares was recently inducted into the UNMC Interprofessional Academy of Educators. She also recently was awarded the ACTG Network Minority HIV Investigator Mentoring Award.

 What a terrific group of talented and accomplished individuals. Kudos to our faculty and staff for their continued hard work and dedication to advancing academic Infectious Diseases!
What a terrific group of talented and accomplished individuals. Kudos to our faculty and staff for their continued hard work and dedication to advancing academic Infectious Diseases! The Rapid Prediction of Carbapenem Resistance in Patients With Klebsiella pneumoniae Bacteremia Using Electronic Medical Record Data.
The Rapid Prediction of Carbapenem Resistance in Patients With Klebsiella pneumoniae Bacteremia Using Electronic Medical Record Data. Using Patient Risk Factors to Identify Whether Carbapenem-Resistant Enterobacteriaceae Infections Are Caused by Carbapenemase-Producing Organisms
Using Patient Risk Factors to Identify Whether Carbapenem-Resistant Enterobacteriaceae Infections Are Caused by Carbapenemase-Producing Organisms The following snippets are Notes from the Field published in MMWR with new information about CRE organisms:
The following snippets are Notes from the Field published in MMWR with new information about CRE organisms: 

 Is there more neuroinvasive disease than in the past? Neuroinvasive disease (e.g., meningitis, encephalitis, or acute flaccid paralysis) occurred in about 46% of West Nile Virus infections
Is there more neuroinvasive disease than in the past? Neuroinvasive disease (e.g., meningitis, encephalitis, or acute flaccid paralysis) occurred in about 46% of West Nile Virus infections  How do I test for it? Should I re-test? In general, diagnosis of West Nile Virus requires testing blood for antibodies to the virus. IgM antibodies present indicate a current or recent infection. If a person has neurologic symptoms (concerning for neuroinvasive disease), lumbar puncture is recommended with testing of the cerebrospinal fluid for West Nile IgM. If the initial IgM test is negative but suspicion for West Nile Virus is high, the antibody test should be repeated in 10 days (Convalescent testing), particularly if symptoms persist.
How do I test for it? Should I re-test? In general, diagnosis of West Nile Virus requires testing blood for antibodies to the virus. IgM antibodies present indicate a current or recent infection. If a person has neurologic symptoms (concerning for neuroinvasive disease), lumbar puncture is recommended with testing of the cerebrospinal fluid for West Nile IgM. If the initial IgM test is negative but suspicion for West Nile Virus is high, the antibody test should be repeated in 10 days (Convalescent testing), particularly if symptoms persist. Can it be prevented? There is no vaccine for West Nile Virus. You can prevent West Nile infection by preventing mosquito bites. Use long sleeves, pants and insect repellents such as DEET or Picardin. Check out the EPA repellent information to help choose the best option here:
Can it be prevented? There is no vaccine for West Nile Virus. You can prevent West Nile infection by preventing mosquito bites. Use long sleeves, pants and insect repellents such as DEET or Picardin. Check out the EPA repellent information to help choose the best option here:  The
The  These outbreaks are only a small sample of the infectious diseases that are occurring in the world today. The
These outbreaks are only a small sample of the infectious diseases that are occurring in the world today. The  Content courtesy Dr. Angela Hewlett, Director of the Nebraska Biocontainment Unit
Content courtesy Dr. Angela Hewlett, Director of the Nebraska Biocontainment Unit






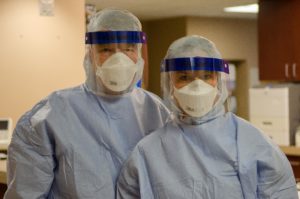 Biocontainment Unit – Opportunities exist for Fellows in the Nebraska Biocontainment Unit (NBU), which is one of only a few units in the United States capable of caring for patients with highly hazardous communicable diseases. The NBU team cared for 3 patients with Ebola Virus Disease in 2014, and has many ongoing efforts to enhance biopreparedness. Fellows have the opportunity to work directly with the Medical Director of the unit, observe and participate in activities in biopreparedness, and possibly participate in patient care activities if the NBU is activated.
Biocontainment Unit – Opportunities exist for Fellows in the Nebraska Biocontainment Unit (NBU), which is one of only a few units in the United States capable of caring for patients with highly hazardous communicable diseases. The NBU team cared for 3 patients with Ebola Virus Disease in 2014, and has many ongoing efforts to enhance biopreparedness. Fellows have the opportunity to work directly with the Medical Director of the unit, observe and participate in activities in biopreparedness, and possibly participate in patient care activities if the NBU is activated.
Recent Comments