Here at UNMC ID, we are thrilled to share the global, and personal efforts, of our faculty. Please take a moment to read this excellent piece by Dr. Nada Fadul; Associate Professor, Division of Infectious Diseases, UNMC

Sudan felt different this time. It was the middle of December 2019, the first anniversary of the Sudanese Revolution that caught the world’s attention. The country is going through a severe economic crisis; bread and gas lines are everywhere, yet the atmosphere is full of hope and enthusiasm.
Our visit was organized by the Association of Sudanese American Professors in America (www.asapa.online). As part of the visit, we toured several universities to conduct informational sessions and needs assessments. We were met with great enthusiasm about the possibility of collaboration with US high education institutions due to sanctions listing Sudan as a State Sponsor of Terrorism (SST) for over 24 years. Being on the SST list prevented Sudan from accessing new technology and knowledge exchange with the US. The needs in the institutions we visited are enormous and the faculty are doing their best with very limited resources. Our agenda at the University of Shendi included a meeting with the Dean of Medicine, the Preisdent of the University, and other faculty, followed by a ceremony celebrating the anniversary of the revolution. I gave a presentation on “Updates in Infectious Diseases” and we had an interesting discussion on the challenges around handwashing, a basic infection control method in the hospital where there is only one sink in the whole floor. The next day we were taken on a tour of the Bajrawia pyramids in the Kingdom of Meroe, one of the ancient Nubian kingdoms, by a graduate of the University of Shendi School of Archeology.The highlight of my trip was our visit to the HIV clinic and the National Tropical Diseases Hospital. On the way there, we passed by the Army Headquarters and I was mesmerized by the revolutionary murals. We arrived at the Tropical Diseases Hospital shortly after noon. The medical director was gracious enough to take time from her busy day to give us a tour of the hospital, their small lab with little microbiology capabilities, and the outpatient clinic. We then proceeded to the Omdurman VCT and ART Center,the largest HIV clinic in the country providing care to over 4,000 patients. The center was a renovated building sponsored by the United Nations Development Program. Sudan is not a recipient of President Emergency Plan for AIDS Relief (PEPFAR) which puts it at a disadvantage when attempting to control the epidemic. The Global Fund provides the antiretroviral medications to the clinic’s pharmacy which then distribute them to patients. The regimens were mostly outdated and limited in options. The HIV clinic lab did not have capabilities for viral load nor CD4 count, making clinical assessment the only way to assess response to antiretrovirals.
In spite of the difficult working environment, the HIV clinic staff is dedicated to making a difference in these patients’ lives. The center is well equipped with highly skilled counselors who conduct needs assessments of patients’ medical, psychosocial, and mental health. All of their assessments are done and stored in paper files as the center does not access to an electronic medical record. There is a dedicated pediatrics clinic space and waiting room as well as a dedicated pediatrics counselor. Several needs were identified including staff training and capacity building, assistance with equipment, and assistance with access to US agencies funding programs for comprehensive HIV care and prevention. Stigma was identified as a major challenge to retaining patients in HIV care as well as concerns about confidentiality.
We concluded our trip with a 1-day conference “Towards Sustainable Development in Sudan: Challenges and Opportunities” attended by the Ministers of Health, Economics, and Irrigation. The Minister of Health delivered a presentation on his transitional period health strategy, including an outbreak response and improving access to preventive services. He emphasized the need to address health disparities in the country, especially war-torn regions in Darfur and Nuba Mountains among other regions. I followed with a presentation on “How Research can Inform Healthcare Policy” and emphasized the role of implementation and dissemination science as well as health outcomes research in the next phase of health transformation. The conference provided a great opportunity for networking with faculty from different universities and staff from the Minister of Health.
Overall, our visit was successful and we were very inspired to see a new Sudan that is full of hope for a bright future. The economy is in bad shape and the healthcare system is in dire need of help, but I am confident that the dedicated Sudanese inside and outside of Sudan will be able to rebuild the country.



 The Nebraska Medicine Specialty Care Center houses our HIV clinic as well as a hygiene pantry that stocks supplies to help patients who don’t have access to these products, supplementing the medical care patients receive and helping break down some of the barriers to good health. In October, Carly McCulloch, an education graduate student at the College of Saint Mary, organized a hygiene drive to benefit our pantry.
The Nebraska Medicine Specialty Care Center houses our HIV clinic as well as a hygiene pantry that stocks supplies to help patients who don’t have access to these products, supplementing the medical care patients receive and helping break down some of the barriers to good health. In October, Carly McCulloch, an education graduate student at the College of Saint Mary, organized a hygiene drive to benefit our pantry.

 One of the most important recommendations related to the treatment of suspected UTI is about using an “Active Monitoring” protocol for those residents who do not meet clinical criteria for UTI and do not have any warning signs (such as fever, rigors, acute delirium, or unstable vital signs), but for whom clinical concern for UTI still exist. Such a protocol include frequent monitoring of vital signs, addressing hydration status and repeated clinical assessments by nursing home staff. Use of such protocols has the potential to decrease inappropriate prescribing for suspected UTI. An example of an active monitoring order set is also included in the document.
One of the most important recommendations related to the treatment of suspected UTI is about using an “Active Monitoring” protocol for those residents who do not meet clinical criteria for UTI and do not have any warning signs (such as fever, rigors, acute delirium, or unstable vital signs), but for whom clinical concern for UTI still exist. Such a protocol include frequent monitoring of vital signs, addressing hydration status and repeated clinical assessments by nursing home staff. Use of such protocols has the potential to decrease inappropriate prescribing for suspected UTI. An example of an active monitoring order set is also included in the document. In short, the AMDA UTI Consensus Statement is a comprehensive guidance document for management of UTI in older adults residing in post-acute and long-term care setting. The document provides guidance to various healthcare professionals including clinicians, administrators, infection preventionists and quality program leaders. The complete document (including the supplementary summary of the recommendations) can be downloaded from the following link
In short, the AMDA UTI Consensus Statement is a comprehensive guidance document for management of UTI in older adults residing in post-acute and long-term care setting. The document provides guidance to various healthcare professionals including clinicians, administrators, infection preventionists and quality program leaders. The complete document (including the supplementary summary of the recommendations) can be downloaded from the following link  Dr. Angela Hewlett, Medical Director of the Nebraska Biocontainment Unit, recently co-authored an article that appeared in the AMA Journal of Ethics entitled; “
Dr. Angela Hewlett, Medical Director of the Nebraska Biocontainment Unit, recently co-authored an article that appeared in the AMA Journal of Ethics entitled; “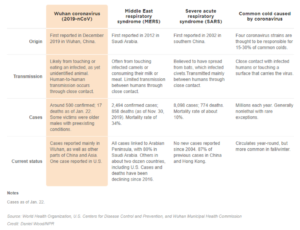
 Content written by Dr. Mark Rupp based on recent publication release:
Content written by Dr. Mark Rupp based on recent publication release:
Recent Comments