As our institution, state, country, and the world grapple with the impacts of SARS-CoV-2, causing COVID-19, there are lots of ongoing discussions about coronaviruses. Dr. Raquel Lamarche is a PGY1 Internal Medicine/Pediatrics resident at UNMC, who will be sharing her thoughts and information she learns about COVID-19. You can follow Dr. Lamarche on Twitter @LamarcheRaquel. This week Dr. Lamarche discusses a pediatric Infectious Diseases primer on SARS-CoV-2.
Children are not tiny adults; thus, it is not surprising that many infectious diseases affect children differently from adults.
We don’t know why COVID-19 appears to be less frequent and severe in children compared to adults. Some considerations include a less vigorous immune response to the virus in children, potential viral interference in the respiratory tract of young children leading to a lower viral load, and perhaps the receptor for SARS-CoV-2, the angiotensin-converting enzyme 2 receptor, is expressed differently in the respiratory tract of children compared to adults. As the pandemic progresses, we expect to learn more from data being published.
Some Epidemiology
• In a case series of >2000 children from China, there was no statistically significant difference in incidence between girls and boys; another case series reported a higher COVID-19 case rate in boys (61%) compared with girls (39%)
• It appears that children are less affected by COVID-19 than adults. This could reflect a lack of widespread availability for SARS-CoV-2 testing or the fact that children are less likely to be tested due to milder disease.
• Coinfection of SARS-CoV-2 and multiple respiratory pathogens can occur in children
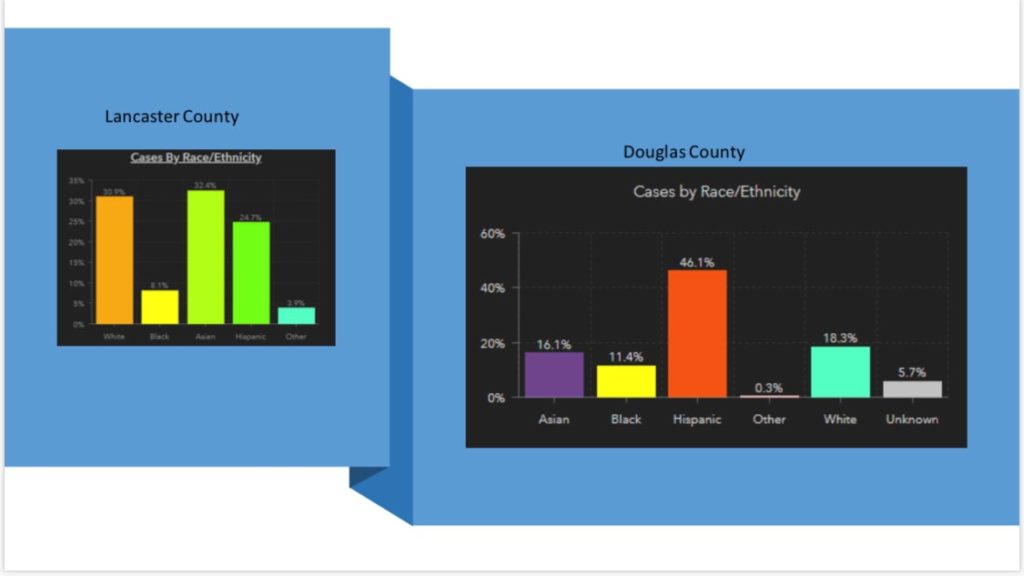
• Children account for 1 to 5 percent of diagnosed COVID-19 cases.
Transmission
 Large droplets: this type of transmission can be prevented using face masks
Large droplets: this type of transmission can be prevented using face masks- Fomites: (objects on which the virus containing droplets have settled) the virus may remain on surfaces for up to 4 days.
- Aerosols: it appears that the virus may also be aerosolized, with a UNMC study finding evidence of (noninfectious) viral particles in the air
Pregnancy and newborns
- Data in pregnancy is minimal. The most extensive case series had 38 cases
- Currently, the virus is not definitively known to be transmitted vertically.
- Of note, there are isolated cases of potential vertical transmission as demonstrated by baby’s elevated IgM against SARS-CoV-2 (IgM does not cross the placenta). The latter is relevant because infants seem to be one of the most vulnerable groups for severe disease in the pediatrics population.
- SARS-CoV-2 does not seem to be transmitted through breast milk. However, droplet transmission can occur through close contact during feeding.
- Healthy pregnant women seem to have the same risk as adults who are not pregnant; however, the CDC warns that contracting the coronavirus while pregnant could make you more vulnerable to severe respiratory problems. This is because pregnant women already have a physiologic restrictive lung disease and relative immunocompromised state.
- One unknown is the impact on women who get sick early in pregnancy and their developing fetus. This is a new virus and nobody who was in the first trimester when they developed COVID-19 has delivered yet.
Symptoms and signs in children
In case series of >2000 children from China:
• 4% of virologically confirmed cases had asymptomatic infection [this rate could be underestimating the accurate scale of asymptomatic infection because many asymptomatic children are unlikely to be tested. On the other hand, children with congenital and chronic diseases are living longer in the US, which means we might have a larger population that is potentially vulnerable to symptomatic and severe disease]
• Among symptomatic children, 5% had dyspnea or hypoxemia, and 0.6% progressed to acute respiratory distress syndrome.
• Some children presented with only gastrointestinal symptoms
• It appears that children generally have a significantly milder disease
One caveat about this observational study:
• Out of the 2135 cases, 66% were “suspected cases” (not test confirmed) defined as a child who was exposed to COVID-19 within the last 2-weeks, or lived in an epidemic area, had 2 of the following conditions: (1) fever, respiratory, digestive symptoms (eg, vomiting, nausea, and diarrhea), or fatigue; (2) laboratory test white blood cell count was normal, decreased, or had a lymphocyte count or increased level of C-reactive protein; or (3) abnormal chest radiograph imaging result. Children often get sick multiple times per year with many of the above findings, completely unrelated to COVID-19 – could some of these “suspected cases” have had symptoms caused by other viral illnesses?
In a US case-series (n=296) the most common symptoms in children were:
• Fever (56%), Cough (54%), and Shortness of breath (13%)
• Less common symptoms: myalgia, fatigue, sore throat, rhinorrhea, nasal congestion, headache, diarrhea, and vomiting
• 73% of pediatric patients had symptoms of either fever, cough, or shortness of breath compared with 93% of adults aged 18–64 years
 In another series of 171 children with confirmed SARS-CoV-2 infection,
In another series of 171 children with confirmed SARS-CoV-2 infection,
• 42% had fever, median duration of 3 days (range 1-16 days)
• 49% had cough, and 16% were asymptomatic
• 19% had upper respiratory infection, but 65% had pneumonia.
• 29% had tachypnea, 42% had tachycardia on admission, and 2.3% had O2 sat <92% during hospitalization
• 77% of children with COVID-19 were in contact with a family member with confirmed SARS-CoV-2
Newborns and infants
In young infants, SARS-CoV-2 can cause fever without any other manifestations, including respiratory symptoms and signs. A 3-week old baby boy who tested positive for SARS-CoV-2 and developed hypercarbic respiratory failure. He had an otherwise negative sepsis workup.
Deaths in children with COVID-19
Although severe cases of COVID-19 in children, including fatal cases, have been reported, most children appear to have a mild or moderate disease and recover within one to two weeks of disease onset.
Laboratory findings
Laboratory findings in children with confirmed infection from Wuhan were variable.
25% had white blood cell count <5.5 x 109/L (5500/microL)
3.5 % had lymphocyte count 46 pg/mL)
20% had elevated C-reactive protein was elevated (>10 mg/L)
Radiographic findings
Radiographic findings may be present before symptoms. CT chest abnormalities noted were:
33% ground-glass opacity
19% patchy local shadowing
12% bilateral patchy shadowing
2% interstitial abnormalities
Risk factors for severe disease
• It appears that infants <1 year of age and children with certain underlying severe conditions are at higher risk for severe disease.
The most commonly reported underlying conditions were:
• Chronic pulmonary disease like asthma
• Cardiovascular disease
• Immunosuppression (cancer, chemotherapy, radiation therapy, hematopoietic cell or solid organ transplant, high doses of glucocorticoids)
• Based on data extrapolated from adults, other medical conditions that may increase the risk of severe disease in children include CKD undergoing dialysis, chronic liver disease, pregnancy, diabetes mellitus, and severe obesity.
 Many of these risk factors are similar to adults. But while much of the conclusions about risk may be extrapolated from adults, children still appear to be affected differently than adults, and this is probably a good reason why more widespread testing, especially in children, may be a good idea, particularly as we look toward the fall and reopening of schools.
Many of these risk factors are similar to adults. But while much of the conclusions about risk may be extrapolated from adults, children still appear to be affected differently than adults, and this is probably a good reason why more widespread testing, especially in children, may be a good idea, particularly as we look toward the fall and reopening of schools.





















Recent Comments