In July 2021 the Center for Disease Control and Prevention (CDC) released their sexually transmitted infections (STI) treatment guideline, an update from 2015.1 Below, senior ID fellow Dr. Jonathan Ryder highlights significant (but by no means comprehensive) changes in this new guideline that can be incorporated into clinical practice and some of the evidence supporting these changes. Part 1 – Chlamydia, Gonorrhea, Mycoplasma genitalium:
Chlamydia
Preferred treatment regimen for chlamydia is now oral doxycycline 100mg twice per day for 7 days. Oral azithromycin 1g for a single dose is now an alternative regimen instead of a preferred regimen.
There are multiple reasons for this change. First, several randomized controlled trials have shown superiority of doxycycline to azithromycin for treatment of chlamydia, including two trials in men who have sex with men (MSM) with rectal chlamydia and one trial in adolescents with urogenital chlamydia.2-4 Second, there is increasing concern for azithromycin resistance in Neisseria gonorrhea, Streptococcus pneumoniae, Mycoplasma genitalium, Shigella, and Campylobacter infections; thus, by changing recommended therapies, we can reduce the selective pressure on these other pathogens.5
There are a few exceptional instances in which a single dose of azithromycin is preferable to a week of doxycycline: pregnancy and concern for nonadherence.
Gonorrhea
Prior guidelines recommended treatment of gonorrhea with a single dose of intramuscular ceftriaxone 250mg plus a single dose of oral azithromycin 1g. In late 2020 the CDC released a new update in Morbidity and Mortality Weekly Report (MMWR), which is now reflected in the current guidelines.5 The new recommended treatment for uncomplicated gonococcal infections of any site is a single dose of intramuscular ceftriaxone 500mg. However, if the patient weighs ³150kg (300 lb), then 1g of intramuscular ceftriaxone should be administered instead.
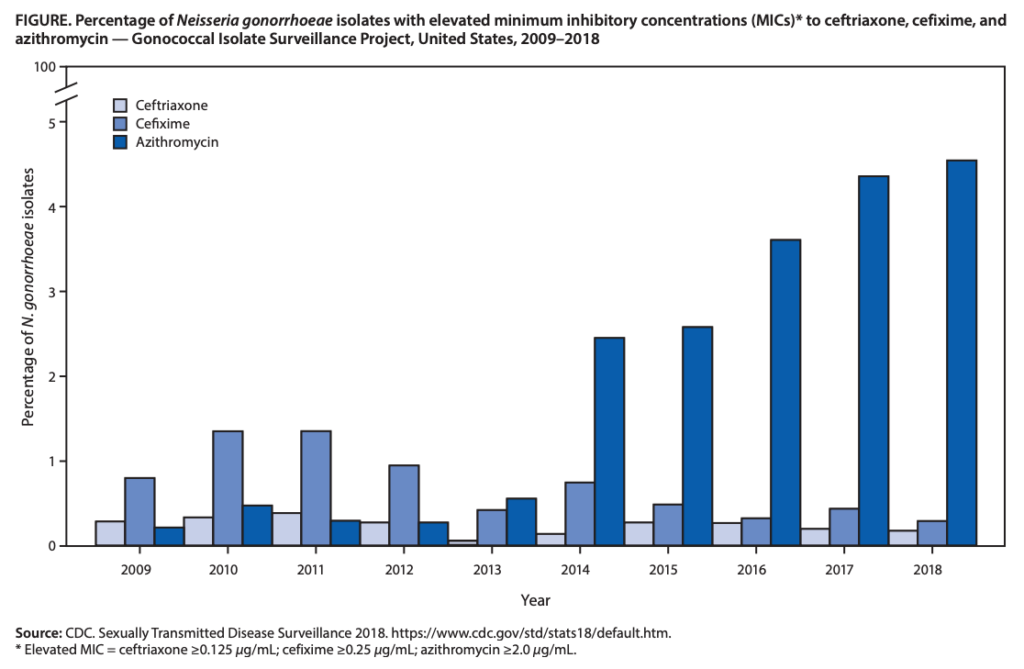
The change in therapy for gonococcal infections is due to emerging resistance, antimicrobial stewardship concerns, and pharmacologic reasons. As shown in the figure below, azithromycin resistance in gonorrhea has increased nearly tenfold from 2013 (0.6%) to 2019 (5.1%).5 As mentioned above, continued azithromycin use has raised concerns in antimicrobial stewardship due to increasing resistance in other pathogens as well as its decreased effectiveness against chlamydia. Lastly, higher doses of ceftriaxone are more likely to achieve adequate time above the MIC, especially in the pharynx, a location known to be more difficult to eradicate N. gonorrhoeae, and in gonococcal strains with higher MICs.
Alternative regimens for gonorrheal infections now include higher dose oral cefixime (800mg for one dose instead of 400mg) as well as intramuscular gentamicin 240mg for one dose combined with a single dose of oral azithromycin 2g. These alternative regimens are not considered reliable for pharyngeal gonorrhea.
Check out this episode from the National STD Curriculum podcast for more on the gonorrhea update: https://www.std.uw.edu/podcast/episode/hot-topic/gonorrhea-treatment-updates
Mycoplasma genitalium
A new development for M. genitalium is the presence of an FDA approved nucleic acid amplification test for multiple specimen types. Testing for M. genitalium should be performed in men with recurrent non-gonoccocal urethritis and women with recurrent cervicitis or pelvic inflammatory disease (PID). Asymptomatic screening is not yet advised due to uncertain clinical benefit.
Treatment for M. genitalium has also changed since prior guidelines due to increasing azithromycin resistance. If resistance testing for macrolide susceptibility is available and performed, that should dictate the treatment regimen. If macrolide sensitive M. genitalium, then oral doxycycline 100mg twice per day for 7 days followed by oral azithromycin 1g first dose then 3 additional days of 500mg daily should be given. However, if macrolide resistant or if susceptibility testing cannot be performed, then a 7-day course of oral doxycycline 100mg twice daily should be followed by oral moxifloxacin 400mg once daily for 7 days.
For more on this topic, the National STD Curriculum podcast also covered M. genitalium: https://www.std.uw.edu/podcast/episode/literature-review/mycoplasma-genitalium
References
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. Published 2021 Jul 23. doi:10.15585/mmwr.rr7004a1
- Lau A, Kong FYS, Fairley CK, et al. Azithromycin or Doxycycline for Asymptomatic Rectal Chlamydia trachomatis. N Engl J Med. 2021;384(25):2418-2427. doi:10.1056/NEJMoa2031631
- Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline Versus Azithromycin for the Treatment of Rectal Chlamydia in Men Who Have Sex With Men: A Randomized Controlled Trial [published online ahead of print, 2021 Feb 19]. Clin Infect Dis. 2021;ciab153. doi:10.1093/cid/ciab153
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus Doxycycline for Urogenital Chlamydia trachomatis Infection. N Engl J Med. 2015;373(26):2512-2521. doi:10.1056/NEJMoa1502599
- St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911-1916. Published 2020 Dec 18. doi:10.15585/mmwr.mm6950a6
