BLASTing Inappropriate Allergies out of the EMR with Antimicrobial Stewardship
 The following is a review by one of our fellows Dr. Rajendra Karnatak from our last Journal Club, who discussed the article by Leis et al: Point-of-Care β-Lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1059–1065.
The following is a review by one of our fellows Dr. Rajendra Karnatak from our last Journal Club, who discussed the article by Leis et al: Point-of-Care β-Lactam Allergy Skin Testing by Antimicrobial Stewardship Programs: A Pragmatic Multicenter Prospective Evaluation, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1059–1065.
There is mounting evidence suggesting patients with labelled allergy to beta-lactams often have worse outcomes, due to receiving second line, less efficacious, broader spectrum antimicrobial agents, and have increased mortality and length of stay. Broad spectrum antimicrobial agents also contribute to development of antimicrobial resistance. Evidence supports most patients with reported allergy to a beta-lactam can safely tolerate beta-lactams. The Infectious Disease Society of America (IDSA) and Society of Healthcare Epidemiology of America (SHEA) recommend that patients with reported beta lactam allergy should undergo beta lactam allergy skin testing. Beta-lactam allergic skin testing is an inexpensive method that can safely exclude type I hypersensitivity reaction with negative predictive value of 97-99%. Beta-lactam testing however, is generally performed by Allergy specialists, and data describing experiences with Antimicrobial Stewardship (ASP) -led allergy testing to de-label allergies is  accumulating slowly.
accumulating slowly.
Beta-Lactam Allergy Skin Testing (BLAST) is a multicenter, prospective study conducted in three centers in Toronto, Canada. In this study, the investigators evaluated the feasibility of point of care beta-lactam allergy skin testing as an ASP activity. There were 3 major objectives of this study: 1) Feasibility of point of care BLAST as an antimicrobial stewardship activity 2) Impact of BLAST on the use of beta-lactams and 3) Impact of BLAST on overall patient clinical outcomes.
At all 3 centers BLAST was performed by ASP teams consisting of an infectious disease physician and antimicrobial stewardship pharmacists. ASP teams at each hospital received drug safety training and hands on training sessions with an Allergist on how to perform and interpret BLAST. During the study period a total of 827 patients with reported allergy to beta-lactams were reviewed by ASP/ID service; in 76% of these patient’s beta lactams were considered preferred agents.
 Based on the beta-lactam allergy history 50% of the patients received preferred beta-lactam agents during the baseline period. This number was increased to 60% during intervention period by careful evaluation by ASP team by history alone (P = .02). During the intervention period after implementation of BLAST, use of preferred beta lactam therapy further increased to 81% (P < .001). The authors concluded there were 4.5-folds higher odds of receiving preferred beta lactam therapy after implementation of BLAST without increase in side effects (95% CI, 2.4–8.2; P < .0001). During the intervention period use of agents with higher risk for C difficile infection such as fluoroquinolones and carbapenems decreased more than half (28% vs 13%; P < .0002) and penicillin use tripled (11% vs 32%; P < .0002).
Based on the beta-lactam allergy history 50% of the patients received preferred beta-lactam agents during the baseline period. This number was increased to 60% during intervention period by careful evaluation by ASP team by history alone (P = .02). During the intervention period after implementation of BLAST, use of preferred beta lactam therapy further increased to 81% (P < .001). The authors concluded there were 4.5-folds higher odds of receiving preferred beta lactam therapy after implementation of BLAST without increase in side effects (95% CI, 2.4–8.2; P < .0001). During the intervention period use of agents with higher risk for C difficile infection such as fluoroquinolones and carbapenems decreased more than half (28% vs 13%; P < .0002) and penicillin use tripled (11% vs 32%; P < .0002).
Although this study was underpowered to evaluate overall clinical outcomes in term of mortality and cost between two groups, investigators were able to demonstrate feasibility of BLAST as an ASP activity. Potential for reducing the use of fluoroquinolones and carbapenems to more than half demonstrates need for a wider integration of BLAST in ASP.
 Patients hospitalized in intensive care units (ICU) can have an 8-times higher likelihood of negative histamine control testing. Given that 25% of their patients were in the intensive care unit (ICU), it was surprising that this study described such a low percentage (4%) of histamine control-negative individuals. It would be interesting to know if the results are as beneficial in ICU settings with higher rates of negative controls, as this might potentially be a group of patients where targeted testing and use of beta-lactams appropriately may have more clinical impact.
Patients hospitalized in intensive care units (ICU) can have an 8-times higher likelihood of negative histamine control testing. Given that 25% of their patients were in the intensive care unit (ICU), it was surprising that this study described such a low percentage (4%) of histamine control-negative individuals. It would be interesting to know if the results are as beneficial in ICU settings with higher rates of negative controls, as this might potentially be a group of patients where targeted testing and use of beta-lactams appropriately may have more clinical impact.
Despite the fact that ASPs are being widely mandated in the United States, there is limited availability of expertise in Allergy and BLAST. Implementation of BLAST as a stewardship activity could provide a much-needed solution to this problem, and by including both academic and community hospitals in their study, the authors have demonstrated that it is feasible. Others have also demonstrated integration of BLAST into ASP, with involvement of pharmacists and ID fellows. Despite its benefits, BLAST is labor-intensive and needed an hour per intervention during this study. Therefore, resources to support BLAST as an ASP activity will need to be addressed before such changes can be implemented.
Editorial Food For Thought: The authors included community and academic hospitals, but all three hospitals are teaching institutions. In truly resource-limited settings like rural non-teaching hospitals, critical access hospitals or those in developing countries, is the benefit worth the expenditure of personnel, materials and time required for this to be successful?  Given the improvement in use of beta-lactams with just more intensive allergy history-taking, is the cost-effective solution simply just doing a better job at allergy review? In many patients it is difficult to ascertain whether the “allergy” (even if real) could really be IgE-mediated, and in this case, BLAST seems to be an ideal solution. One clinical situation where we have clear evidence of differences in clinical outcomes with beta-lactams vs alternatives is S. aureus bacteremia; are these the targeted clinical syndromes where BLAST would be most cost-effective, especially in a resource-limited setting?
Given the improvement in use of beta-lactams with just more intensive allergy history-taking, is the cost-effective solution simply just doing a better job at allergy review? In many patients it is difficult to ascertain whether the “allergy” (even if real) could really be IgE-mediated, and in this case, BLAST seems to be an ideal solution. One clinical situation where we have clear evidence of differences in clinical outcomes with beta-lactams vs alternatives is S. aureus bacteremia; are these the targeted clinical syndromes where BLAST would be most cost-effective, especially in a resource-limited setting?

 Olivia: During my studies at UNL, I became interested in underserved communities after volunteering at the People’s City Mission (PCM) free health clinic. While at PCM, I interacted with patients who fell through the cracks in our healthcare system, a system that I have always been able to access. Soon after, I began to recognize disparity in my hometown of Columbus, NE in patients living in rural areas who struggle to meet with urban specialists to manage their health problems. Furthermore, my major of Global Studies took me to Mumbai, India where I met patients diagnosed with epilepsy who faced a great deal of social stigma surrounding their disease.
Olivia: During my studies at UNL, I became interested in underserved communities after volunteering at the People’s City Mission (PCM) free health clinic. While at PCM, I interacted with patients who fell through the cracks in our healthcare system, a system that I have always been able to access. Soon after, I began to recognize disparity in my hometown of Columbus, NE in patients living in rural areas who struggle to meet with urban specialists to manage their health problems. Furthermore, my major of Global Studies took me to Mumbai, India where I met patients diagnosed with epilepsy who faced a great deal of social stigma surrounding their disease. Rohan: My interest in health disparities largely stems from the time I spent as an undergraduate in St. Louis. Following the shooting of Michael Brown in Ferguson, a neighborhood 10-15 minutes from where I lived at the time, I became acutely aware that St. Louis, like Omaha, is a city with historically ingrained divisions that create disparities in the social determinants of its citizens’ health. I was inspired by the widespread activism I saw around St. Louis and involved myself in a narrative-based music outreach program to help uplift the stories of young community members. I was also motivated to leverage my own leadership positions on campus to advocate for the mental health of students of color and LGBTQ+ students.
Rohan: My interest in health disparities largely stems from the time I spent as an undergraduate in St. Louis. Following the shooting of Michael Brown in Ferguson, a neighborhood 10-15 minutes from where I lived at the time, I became acutely aware that St. Louis, like Omaha, is a city with historically ingrained divisions that create disparities in the social determinants of its citizens’ health. I was inspired by the widespread activism I saw around St. Louis and involved myself in a narrative-based music outreach program to help uplift the stories of young community members. I was also motivated to leverage my own leadership positions on campus to advocate for the mental health of students of color and LGBTQ+ students. Laura: Through volunteering at community organizations growing up, I became aware of differences in wealth, educational opportunities, and neighborhood resources between Omaha communities. During a college course in Ecuador which focused on social and political transformations, I gained a broader understanding of the global pervasiveness of inequalities, especially in health. In Minnesota where I attended college, I volunteered at a community health clinic teaching an exercise and nutrition class for Hispanic and Somali women struggling with obesity and diabetes. The hard work, persistence, and camaraderie of these women left me inspired and grateful for their friendship and the privilege to take part in their path to better health, and I felt drawn to a vocation in health care.
Laura: Through volunteering at community organizations growing up, I became aware of differences in wealth, educational opportunities, and neighborhood resources between Omaha communities. During a college course in Ecuador which focused on social and political transformations, I gained a broader understanding of the global pervasiveness of inequalities, especially in health. In Minnesota where I attended college, I volunteered at a community health clinic teaching an exercise and nutrition class for Hispanic and Somali women struggling with obesity and diabetes. The hard work, persistence, and camaraderie of these women left me inspired and grateful for their friendship and the privilege to take part in their path to better health, and I felt drawn to a vocation in health care. Independence Day is the
Independence Day is the  Hot Dogs/Deli meat: Staphylococcus aureus – causes nausea, vomiting, diarrhea, cramps within 30 minutes-6 hours
Hot Dogs/Deli meat: Staphylococcus aureus – causes nausea, vomiting, diarrhea, cramps within 30 minutes-6 hours Burgers: Escherichia coli – causes diarrhea, stomach cramps, vomiting within 3-4 days
Burgers: Escherichia coli – causes diarrhea, stomach cramps, vomiting within 3-4 days Step 1 – Clean: Wash your hands, utensils and food handling surfaces often
Step 1 – Clean: Wash your hands, utensils and food handling surfaces often Step 4 – Chill: Refrigerate your food promptly, and at most within 2 hours (within 1 hour if outside temperature is above 90°F). After cooking, put the food back in the cool refrigerator and make sure it can keep temperatures below 40°F. Food left at room temperature for long periods invites bacteria to multiply rapidly, increasing likelihood of foodborne illnesses.
Step 4 – Chill: Refrigerate your food promptly, and at most within 2 hours (within 1 hour if outside temperature is above 90°F). After cooking, put the food back in the cool refrigerator and make sure it can keep temperatures below 40°F. Food left at room temperature for long periods invites bacteria to multiply rapidly, increasing likelihood of foodborne illnesses. Most importantly, WASH YOUR HANDS before and after handling raw meat, before handling non-meat items, and before you sit down to enjoy the fruit of your grilling prowess. (Or at least use hand sanitizer!)
Most importantly, WASH YOUR HANDS before and after handling raw meat, before handling non-meat items, and before you sit down to enjoy the fruit of your grilling prowess. (Or at least use hand sanitizer!) In 2006, the Centers for Disease Control and Prevention (CDC) recommended universal HIV screening, which was endorsed by the United States Preventive Services Taskforce (USPSTF) in 2013. Despite these recommendations, 1 in 7 persons living with HIV (PLWH) in the United States are unaware of their diagnosis. When stratified by age, young people between the ages of 13-24 account for
In 2006, the Centers for Disease Control and Prevention (CDC) recommended universal HIV screening, which was endorsed by the United States Preventive Services Taskforce (USPSTF) in 2013. Despite these recommendations, 1 in 7 persons living with HIV (PLWH) in the United States are unaware of their diagnosis. When stratified by age, young people between the ages of 13-24 account for  Our Specialty Care Clinic works closely with the Douglas County STD Clinic and NAP. We treat PLWH diagnosed with HIV at these locations and provide pre-exposure prophylaxis (PrEP) for patients who are at risk. Our nurse and case manager Precious Davis BSN, MSN has been very involved with reaching out to our community to spread the message of HIV testing. At an event in the Spring, Precious shared some of these statistics and information on treatment and stigma of HIV care in a poem (pictured here). At that event, 25 people were tested for HIV.
Our Specialty Care Clinic works closely with the Douglas County STD Clinic and NAP. We treat PLWH diagnosed with HIV at these locations and provide pre-exposure prophylaxis (PrEP) for patients who are at risk. Our nurse and case manager Precious Davis BSN, MSN has been very involved with reaching out to our community to spread the message of HIV testing. At an event in the Spring, Precious shared some of these statistics and information on treatment and stigma of HIV care in a poem (pictured here). At that event, 25 people were tested for HIV.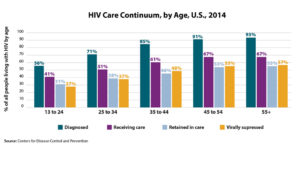 The
The  Then it got worse. Around 1 week later, the cough started. The body aches. The headaches. The sore throat. The “influenza-like illness,” or ILI, arrived with no apologies. I thought the virus had done its worst; then, as I made my morning coffee, Wacky Wednesday started. I could not smell it. It seemed strange, but I thought it was just congestion. I took a sip. Hot liquid, tasteless. I rummaged through my kitchen–smelling and tasting noxious things, testing my senses. Garlic? Nothing. Chili Lime seasoning? Nothing. Pepper, salt, ginger? All nothing.
Then it got worse. Around 1 week later, the cough started. The body aches. The headaches. The sore throat. The “influenza-like illness,” or ILI, arrived with no apologies. I thought the virus had done its worst; then, as I made my morning coffee, Wacky Wednesday started. I could not smell it. It seemed strange, but I thought it was just congestion. I took a sip. Hot liquid, tasteless. I rummaged through my kitchen–smelling and tasting noxious things, testing my senses. Garlic? Nothing. Chili Lime seasoning? Nothing. Pepper, salt, ginger? All nothing. For most infections, think the common cold or upper respiratory infections, it seems that the post-infection loss of smell is generally temporary due to the phenomenal plasticity of the olfactory system. In fact, after URI’s, 32-66% of patients will recover their sense of smell spontaneously. For those struggling, training your sniffer can carry significant advantages toward recovering with intentionally smelling various types of odors a few times per day to “retrain” the olfactory system. It’s physical therapy for the nose!
For most infections, think the common cold or upper respiratory infections, it seems that the post-infection loss of smell is generally temporary due to the phenomenal plasticity of the olfactory system. In fact, after URI’s, 32-66% of patients will recover their sense of smell spontaneously. For those struggling, training your sniffer can carry significant advantages toward recovering with intentionally smelling various types of odors a few times per day to “retrain” the olfactory system. It’s physical therapy for the nose! After almost a year of planning, the First Annual Nebraska Antimicrobial Stewardship Summit convened on Friday, June 1st, 2018 in La Vista, NE. This conference is the first of its kind in Nebraska in which information on antimicrobial stewardship in various healthcare settings is the focus of the meeting. The conference center was abuzz with excitement as close to 270 healthcare professionals attended the Summit that included over 130 nurses, 80 pharmacists, and 30 providers. While the majority of the attendees were from Nebraska, healthcare professionals from neighboring states such as Iowa, Kansas, Missouri and South Dakota also attended the Summit.
After almost a year of planning, the First Annual Nebraska Antimicrobial Stewardship Summit convened on Friday, June 1st, 2018 in La Vista, NE. This conference is the first of its kind in Nebraska in which information on antimicrobial stewardship in various healthcare settings is the focus of the meeting. The conference center was abuzz with excitement as close to 270 healthcare professionals attended the Summit that included over 130 nurses, 80 pharmacists, and 30 providers. While the majority of the attendees were from Nebraska, healthcare professionals from neighboring states such as Iowa, Kansas, Missouri and South Dakota also attended the Summit. The morning session continued with presentations from Dr. Diekema (Professor, University of Iowa Carver College of Medicine, pictured to the left) on the Role of the Laboratory in Antimicrobial Stewardship; Drs. Vivekanandan (Associate Professor of Medicine, Creighton University) and Horne (Assistant Professor of Medicine, Creighton University) on “Is Antibiotic Stewardship the Answer to C. difficile”; and Kate Tyner, RN, CIC (Nurse Coordinator, Nebraska ASAP and ICAP) on the “Role of the Infection Preventionist in Antimicrobial Stewardship”. The morning session concluded with a presentation from Drs. Tierney and Pedati (Medical Epidemiologist, Nebraska DHHS) on “Public Health Support for Antimicrobial Stewardship” in which they discussed the state MDRO outbreak detection and management protocols as well as the state antimicrobial susceptibility registry and antibiogram.
The morning session continued with presentations from Dr. Diekema (Professor, University of Iowa Carver College of Medicine, pictured to the left) on the Role of the Laboratory in Antimicrobial Stewardship; Drs. Vivekanandan (Associate Professor of Medicine, Creighton University) and Horne (Assistant Professor of Medicine, Creighton University) on “Is Antibiotic Stewardship the Answer to C. difficile”; and Kate Tyner, RN, CIC (Nurse Coordinator, Nebraska ASAP and ICAP) on the “Role of the Infection Preventionist in Antimicrobial Stewardship”. The morning session concluded with a presentation from Drs. Tierney and Pedati (Medical Epidemiologist, Nebraska DHHS) on “Public Health Support for Antimicrobial Stewardship” in which they discussed the state MDRO outbreak detection and management protocols as well as the state antimicrobial susceptibility registry and antibiogram. During lunch, Summit attendees had the opportunity for roundtable discussions with
During lunch, Summit attendees had the opportunity for roundtable discussions with  Education in antimicrobial stewardship continues in the afternoon with breakout sessions in the Acute and Ambulatory Track and the Post-Acute and Long-Term Care Track at the Summit. Dr. Bergman (Pharmacy Coordinator, Antimicrobial Stewardship Program, Nebraska Medicine, pictured to the left) started the acute and ambulatory session with his presentation on “Regulatory Requirements for Hospitals and Outpatient Antimicrobial Stewardship”. This was followed by presentations from Dr. Van Schooneveld (Medical Director, Antimicrobial Stewardship Program, Nebraska Medicine) on “Antimicrobial Stewardship Interventions in Acute Care Hospitals”; Dr. Kuper (Senior Clinical Manager, Infectious Diseases, Vizient) on “Antibiotic Stewardship Metrics: How Do You Measure Up?”; and Drs. Marcelin (Associate Medical Director, Antimicrobial Stewardship Program, Nebraska Medicine) and Green Hines (Medical Director, Antimicrobial Stewardship Program, Children’s Hospital & Medical Center) on “Antimicrobial Stewardship in the Outpatient Setting (#OutptASP)”. The session was well attended and appreciated by Summit attendees.
Education in antimicrobial stewardship continues in the afternoon with breakout sessions in the Acute and Ambulatory Track and the Post-Acute and Long-Term Care Track at the Summit. Dr. Bergman (Pharmacy Coordinator, Antimicrobial Stewardship Program, Nebraska Medicine, pictured to the left) started the acute and ambulatory session with his presentation on “Regulatory Requirements for Hospitals and Outpatient Antimicrobial Stewardship”. This was followed by presentations from Dr. Van Schooneveld (Medical Director, Antimicrobial Stewardship Program, Nebraska Medicine) on “Antimicrobial Stewardship Interventions in Acute Care Hospitals”; Dr. Kuper (Senior Clinical Manager, Infectious Diseases, Vizient) on “Antibiotic Stewardship Metrics: How Do You Measure Up?”; and Drs. Marcelin (Associate Medical Director, Antimicrobial Stewardship Program, Nebraska Medicine) and Green Hines (Medical Director, Antimicrobial Stewardship Program, Children’s Hospital & Medical Center) on “Antimicrobial Stewardship in the Outpatient Setting (#OutptASP)”. The session was well attended and appreciated by Summit attendees. Equally well attended is the post-acute and long-term care session that was opened with a presentation from Dr. Crnich (Chief of Medicine and Hospital Epidemiologist, Williams S. Middleton VA Hospital, pictured to the left) on “Regulatory Requirements for Post-Acute and Long-Term Care Antimicrobial Stewardship Programs”. This afternoon session continued with Dr. Ashraf (Co-Medical Director, Nebraska ASAP and Medical Director, Nebraska ICAP) speaking on “Antimicrobial Stewardship Implementation in Post-Acute and Long-Term Care Facilities”; Dr. Crnich on “Management of Common Infections in Long-Term Care Facilities”. The session concluded with a presentation from Tammi Schaffart, RN (Infection Control Nurse and QAPI Coordinator, Douglas County Health Center) and Dr. Ortmeier (Consultant Pharmacist Team Lead, Community Pharmacy Services) on the “Role of Nurses and Consultant Pharmacists in Antibiotic Stewardship in Post-Acute and Long-Term Care Facilities”.
Equally well attended is the post-acute and long-term care session that was opened with a presentation from Dr. Crnich (Chief of Medicine and Hospital Epidemiologist, Williams S. Middleton VA Hospital, pictured to the left) on “Regulatory Requirements for Post-Acute and Long-Term Care Antimicrobial Stewardship Programs”. This afternoon session continued with Dr. Ashraf (Co-Medical Director, Nebraska ASAP and Medical Director, Nebraska ICAP) speaking on “Antimicrobial Stewardship Implementation in Post-Acute and Long-Term Care Facilities”; Dr. Crnich on “Management of Common Infections in Long-Term Care Facilities”. The session concluded with a presentation from Tammi Schaffart, RN (Infection Control Nurse and QAPI Coordinator, Douglas County Health Center) and Dr. Ortmeier (Consultant Pharmacist Team Lead, Community Pharmacy Services) on the “Role of Nurses and Consultant Pharmacists in Antibiotic Stewardship in Post-Acute and Long-Term Care Facilities”. Track shared their thoughts. Dr. Crnich said it takes a team to establish a stewardship program while Dr. Ashraf (pictured to the left) echoed a similar sentiment that no one is alone in this journey. Tammi emphasized to the many nurses in the audience the importance of documentation to show their efforts for the numerous clinical activities they performed in nursing facilities, including antimicrobial stewardship. Dr. Ortmeier stressed the importance of persistence and the need to continue to ‘keep at it’ for eventual success.
Track shared their thoughts. Dr. Crnich said it takes a team to establish a stewardship program while Dr. Ashraf (pictured to the left) echoed a similar sentiment that no one is alone in this journey. Tammi emphasized to the many nurses in the audience the importance of documentation to show their efforts for the numerous clinical activities they performed in nursing facilities, including antimicrobial stewardship. Dr. Ortmeier stressed the importance of persistence and the need to continue to ‘keep at it’ for eventual success. We genuinely appreciate the support Summit attendees expressed. It is our hope that this type of Summit will continue annually in the future and that new topics and updated contents in antimicrobial stewardship will be presented. Additionally, we hope to make many more connections with healthcare professionals in Nebraska and neighboring states to improve the care and safety of our patients and residents by improving prescribing of this precious resource, antimicrobials, through antimicrobial stewardship.
We genuinely appreciate the support Summit attendees expressed. It is our hope that this type of Summit will continue annually in the future and that new topics and updated contents in antimicrobial stewardship will be presented. Additionally, we hope to make many more connections with healthcare professionals in Nebraska and neighboring states to improve the care and safety of our patients and residents by improving prescribing of this precious resource, antimicrobials, through antimicrobial stewardship. PADDLE Trial4: A pilot, non-blinded, non-randomized, non-parallel, 48 week trial in Argentina. This trial enrolled 20 patients that were naïve to ART, viral loads ranging from 5,000 to 100,000 copies/mL and CD4+ greater 200cells/mm3. The participants in this trial were primarily assessed to see the rate of success at achieving HIV levels of 50 copies/mL or less at 48 weeks. At 48 weeks, 18/20 (90%) patients reached the viral levels desired, including 4 patients with viral loads greater than 100,000 (albeit to protocol). Additionally, safety and efficacy of this dual therapy was analyzed in this trial. Only one protocol failure developed; however, participants achieved levels less than 50 copies/mL from Week 4-24 and developed no resistance to any of the agents.
PADDLE Trial4: A pilot, non-blinded, non-randomized, non-parallel, 48 week trial in Argentina. This trial enrolled 20 patients that were naïve to ART, viral loads ranging from 5,000 to 100,000 copies/mL and CD4+ greater 200cells/mm3. The participants in this trial were primarily assessed to see the rate of success at achieving HIV levels of 50 copies/mL or less at 48 weeks. At 48 weeks, 18/20 (90%) patients reached the viral levels desired, including 4 patients with viral loads greater than 100,000 (albeit to protocol). Additionally, safety and efficacy of this dual therapy was analyzed in this trial. Only one protocol failure developed; however, participants achieved levels less than 50 copies/mL from Week 4-24 and developed no resistance to any of the agents. GEMINI I1 and GEMINI II2: Two, nearly identical large phase III, randomized, double-blinded, multinational, multicenter, parallel studies that enrolled 719 and 722 ART naïve participants. The primary purposes of these studies were to assess the percentage of subjects with viral loads between 1,000-100,000 copies/mL and CD4>200/mm3 who achieved viral suppression (HIV VL<50 copies/mL) at Week 48. Preliminary results for these trials are expected to be released in the summer of 2018 and study completion is scheduled for March 2020.
GEMINI I1 and GEMINI II2: Two, nearly identical large phase III, randomized, double-blinded, multinational, multicenter, parallel studies that enrolled 719 and 722 ART naïve participants. The primary purposes of these studies were to assess the percentage of subjects with viral loads between 1,000-100,000 copies/mL and CD4>200/mm3 who achieved viral suppression (HIV VL<50 copies/mL) at Week 48. Preliminary results for these trials are expected to be released in the summer of 2018 and study completion is scheduled for March 2020. Content provided by Freddy Orellana, UNMC PharmD Candidate ’18, and Joshua Havens PharmD
Content provided by Freddy Orellana, UNMC PharmD Candidate ’18, and Joshua Havens PharmD
Recent Comments