The Nebraska HIV Prevention and Care Update on May 8, 2025, brought together healthcare professionals, public health leaders, and community advocates to share the latest developments in HIV care, prevention, and support services. The event emphasized innovation, community-based strategies, and integrated care models. The 2025 HIV Update conference was hosted in person at Mammel Hall at UNO, as well as virtually, and had over 150 attendees in total.
Session Highlights
Co-chairs Andy Dillehay, Gaye Gwion, and Nikki Regan offered opening remarks to set the tone for a collaborative and forward-thinking day focused on improving HIV outcomes across Nebraska. Logan Reynolds (NEDHHS Ryan White Program Coordinator) and Daemon Donigan (AIDS United Specialist) provided updates on the Ryan White HIV/AIDS Program, HOPWA, and capacity-building efforts. They highlighted funding priorities, service delivery improvements, and strategies to enhance provider training and infrastructure.
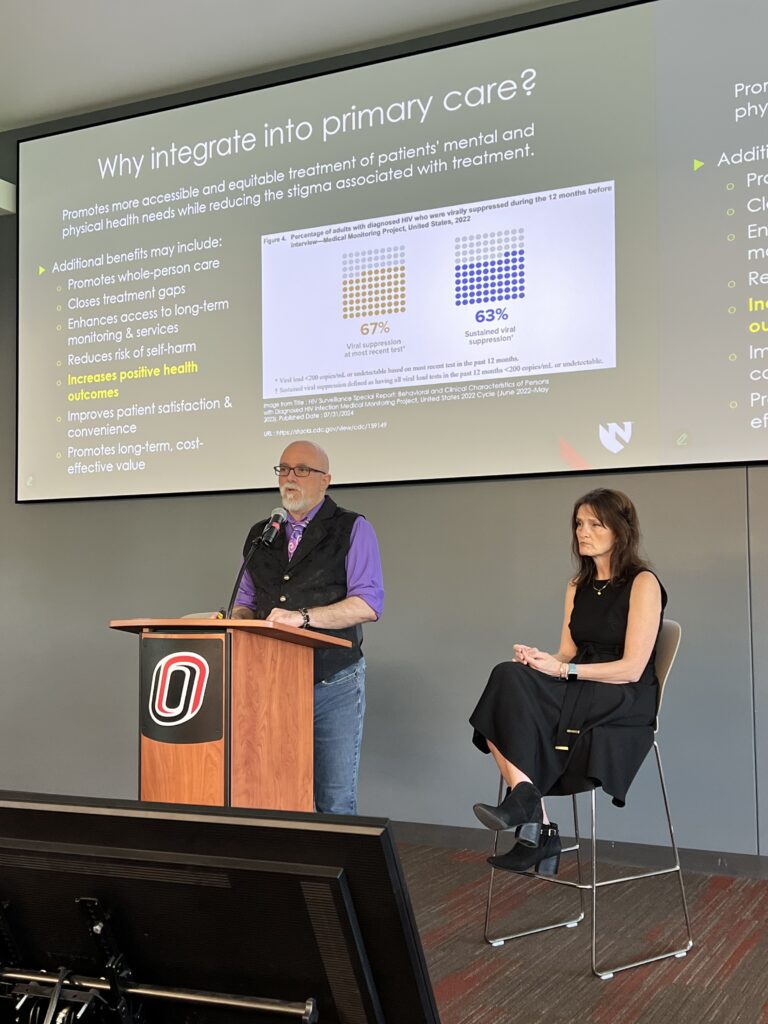
Dr. Sara Bares (UNMC Associate Professor) discussed the evolving landscape of HIV treatment, emphasizing long-acting injectable therapy options. Dr. Jenn Davis (UNMC Assistant Professor) followed with updates on integrating HIV care into primary care settings, stressing the importance of patient-centered approaches to address lifelong co-morbidity risks for patients living longer with HIV.
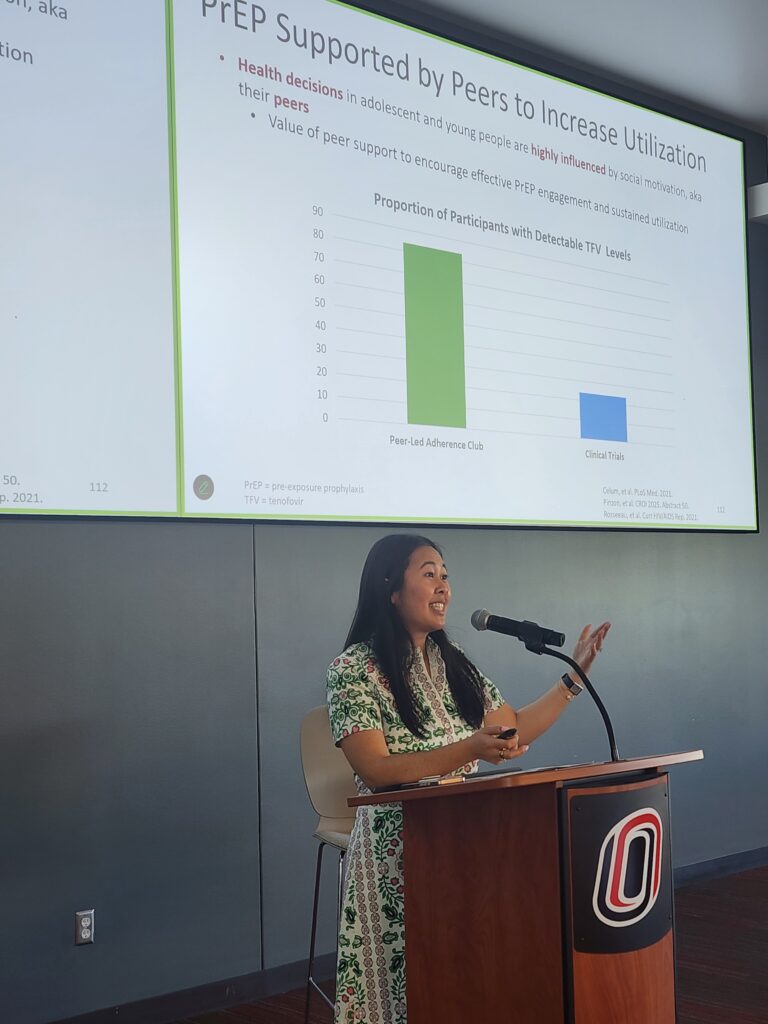
Dr. Josh Havens (HIV program PharmD) introduced next-generation PrEP options, including long-acting injectables and new delivery methods. Dr. Shawna Sunagawa (HIV program PharmD) focused on community-based interventions, highlighting culturally responsive strategies to expand PrEP access, and citing a current outreach effort at One World Community Health Center.


The lunch hour was a chance for in-person attendees to network with others, including several SCC team members who hosted the inaugural HIV Update Poster Hall. Jen O’Neill and Mo Kubat (SCC Cabenuva nurses/program coordinators) shared a poster highlighting current impacts of the IM-CAPABLE study, to expand long-acting injectable antiretroviral therapy to central Nebraska. Emmanuel Essam-Nkodo (SCC Research Coordinator) also shared 2 posters which have been viewed at international conferences, showcasing the impact of Medicaid expansion on HIV outcomes in Nebraska as well as access to COVID-19 treatments for people with HIV in Nebraska.
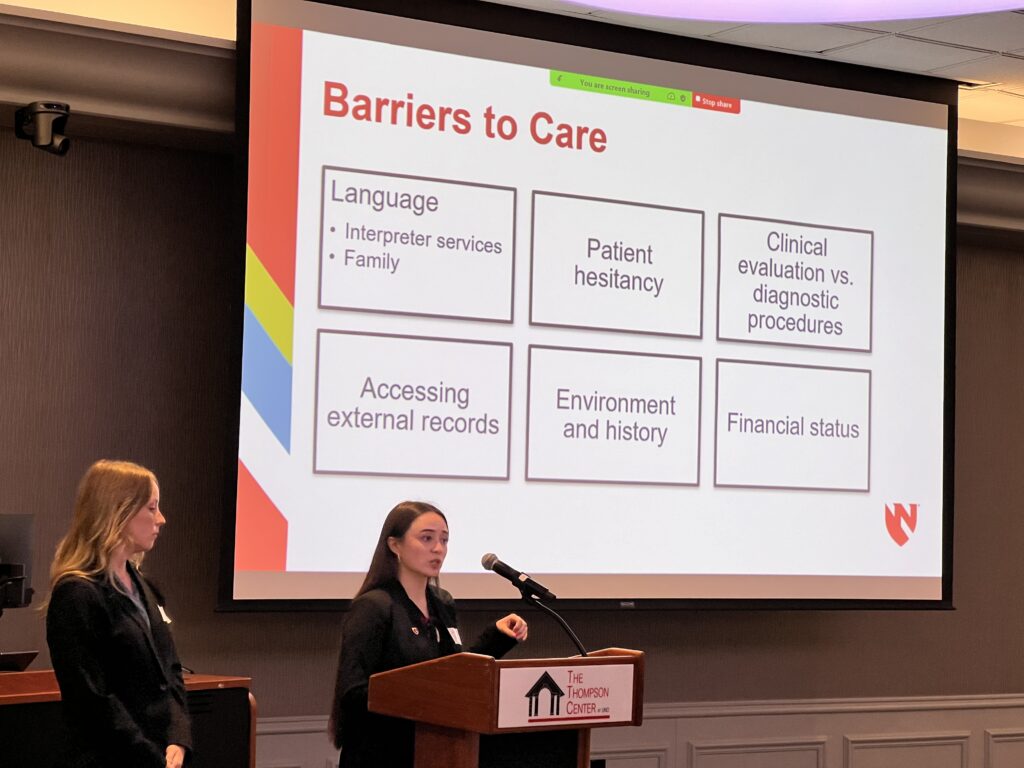
Melissa Neuenfeldt (RN, COO) and Dr. Dalton Nelsen (UNMC, HEAL CEO) presented HEAL Omaha’s street medicine model, which delivers care directly to unhoused and underserved individuals. Their work demonstrates how mobile outreach can bridge gaps in HIV prevention and treatment.
Lance Burwell (SCC Behavioral Therapist) and Renae Furl (SCC Clinical Study Coordinator) explored the intersection of behavioral health and HIV management, advocating for trauma-informed practices to support vulnerable populations and improve engagement in care. They also described the recent successes and lessons learned through the innovative i2TEC project, which supports patients with telehealth based coaching sessions.
Alyssa Maxwell (CenterPointe Program Director) addressed the impact of substance use on HIV transmission and care. She emphasized integrated health teams and supportive services as key to reducing stigma and improving outcomes.
Nikki Regan, Gaye Gwion, and Daemon Donigan concluded the day by reinforcing the importance of collaboration, innovation, and community engagement in the fight against HIV in Nebraska. We thank all our community partners and attendees.

If you are interested in attending future HIV Update conferences, or have suggestions for important topics, please contact Nikki Regan at nregan@nebraskamed.com.




















Recent Comments