This just in! A new article published last month by Dr. Andre Kalil outlines an effective measure to treat COVID-19 in immunocompromised individuals at elevated risk of serious disease from this infection. See below for a quick digest of the article, and read the full story here.
Why is it essential to research COVID-19 treatment specifically in the immunocompromised population?

This population is at elevated risk of severe disease and death resulting from infection. This is due to an increased susceptibility to the virus and the reduced efficacy of preventative measures, such as vaccines. Therefore, these patients have been largely excluded from clinical trials centered around COVID-19 for ethical and logistical reasons. This means there are comparatively few evidence-backed medical standards for treating COVID-19 infection in this population, leaving medical professionals with insufficient standardized guidance on which regimens are safe and effective.
How does the study address this gap in evidence-based medicine?
This study focused specifically on immunocompromised COVID-19 patients and retrospectively assessed whether or not they were administered remdesivir, a common COVID-19 treatment, during their hospital stay, calculating all-cause mortality rates for each population. More than 50,000 patients from 48 US states were assessed in this study.
What is remdesivir?
Remdesivir is an anti-viral medication that has been shown to be very effective in reducing death, disease severity (including the need for assisted ventilation), and hospitalization rates among infected individuals. It works by interfering with the ability of the virus to replicate itself and infect other cells.
What did they find?
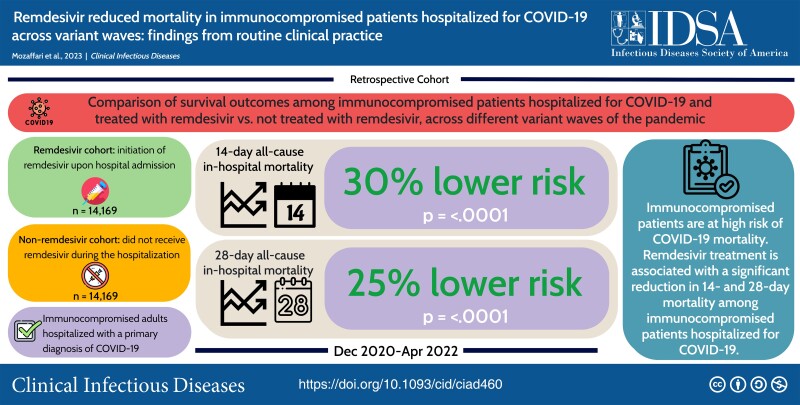
The authors found that patients who were administered remdesivir had a much lower mortality rate than those who did not receive the medication. This trend was consistent across multiple different SARS-CoV-2 variants, including pre-Delta, Delta, and Omicron viruses. The authors concluded, “Remdesivir, with its established efficacy and safety profile and widespread availability, is an important therapeutic option for treatment of COVID-19 in immunocompromised patients“.