
This #PharmToExamTable post was authored by Eric Hiatt, PharmD Candidate (2025) at the UNMC College of Pharmacy, and explores Bactrim’s use in those with impaired renal function.
(Content reviewed by Jenna Preusker, PharmD, BCPS, BCIDP)
In the world of antimicrobial stewardship, finding the right antibiotic for definite therapy against a bacterial infection can be the difference between life and death. Bacterial resistance has emerged with the use and misuse of antibiotics. The result leads to dwindling options and reliance on less-than-ideal antibiotics for specific populations. An example is sulfamethoxazole/trimethoprim in populations with renal impairment or those on dialysis.
A meta-analysis explored the adverse renal effects of sulfamethoxazole/trimethoprim and included patients who initially received their first dose in the hospital. The study included 573 patients, of which 64 (11%) developed an AKI. Out of the 64 with AKI, the authors noted a significantly higher percentage of patients had pre-existing chronic kidney disease. The inclusion criteria itself has a limitation; with or without sulfamethoxazole/trimethoprim, many hospitalized patients are likely to develop an AKI. To control this limitation, the authors included a comparator group with similar characteristics but who were administered clindamycin instead due to no known AKI-associated events with clindamycin. The clindamycin group consisted of 84 patients, of which none developed an AKI, with only 3 having an increase in serum creatinine and no increase in BUN. The study concluded that since there were patients who developed AKI even with no other known risk factors for renal impairment, sulfamethoxazole/trimethoprim was suggested to be an independent risk factor for AKI.1
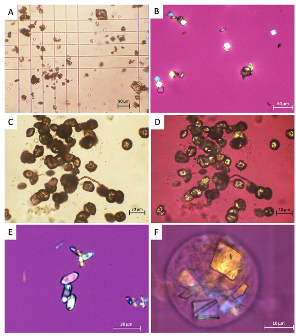
In 2024, a case series of 14 patients investigated whether sulfamethoxazole-induced crystalline structures result in AKI. The crystalline structure results from sulfamethoxazole’s metabolite N-acetyl-sulfamethoxazole (NASM). NASM has been observed to form a crystalline structure in acidic urine, potentially causing renal damage resulting in an AKI. The authors concluded that patients with sulfamethoxazole-induced crystalline nephropathy developed an AKI. They also noted that individuals at risk for crystalline structures forming from NASM include patients who have hypovolemia, hypoalbuminemia, acidic urine, high concentrations of sulfamethoxazole/trimethoprim and NASM in urine, and chronic kidney disease.2
In hospitalized patients, providers can avoid crystallization by giving intravenous normal saline for hydration and promoting diuresis with an agent like a loop diuretic. Another way would be to ensure the pH of the urine is basic (pH > 7.15), as it would increase sulfamethoxazole solubility in the urine, preventing further crystallization. If needed, sodium bicarbonate can be administered to increase urine pH. Additionally, clinicians should monitor the estimated glomerular filtration rate and monitor urine for crystalluria. In acute renal failure resulting from sulfamethoxazole/trimethoprim, the first step is to discontinue it and administer fluids to ensure the patient is euvolemic, followed by diuresis to clear any obstructing crystals within the kidney. If needed, sulfamethoxazole/trimethoprim can also be removed through dialysis.3
To be continued in Part 2 soon!
References:
1. Fraser TN, Avellaneda AA, Graviss EA, et al. Acute kidney injury associated with trimethoprim/sulfamethoxazole. Journal of Antimicrobial Chemotherapy, 2012; 67(5), 1271–1277. https://doi.org/10.1093/jac/dks030
2. Azencot R, Saint-Jacques C, Haymann JP, et al. Sulfamethoxazole-induced crystal nephropathy: Characterization and prognosis in a case series. Scientific Reports, 2024; 14, 6078. https://doi.org/10.1038/s41598-024-56322-9
3. Perazella MA. Crystal-induced acute renal failure. The American Journal of Medicine, 1999; 106(4), 459–465. https://doi.org/10.1016/S0002-9343(99)00041-8
1 comment