
Antibiotic usage frequently has rapidly changing recommendations. This summary reviews the first 4 of the top 8 antibiotic myths facing clinicians. Check back to the next post for the remaining antibiotic-prescribing myths!

Written by Savona Bateman (left)
UNMC PharmD Candidate 2024
Reviewed by Jenna Preusker (right), PharmD, BCPS, BCIDP
Nebraska ASAP Pharmacy Coordinator

McCreary E, Johnson M, Jones T, et al. Antibiotic Myths for the Infectious Diseases Clinician, Clinical Infectious Diseases, Volume 77, Issue 8, 15 October 2023, 1120–1125, https://doi.org/10.1093/cid/ciad357
Myth 1: Cefazolin should be avoided for central nervous system infections
Tertiary medical references commonly state that cefazolin should not be used for meningitis because it does not adequately attain high enough concentrations in the cerebrospinal fluid (CSF) to be effective for central nervous system infections. This statement came from two main studies in the 1970s. The first study in 1973 used another first-generation cephalosporin called cephalothin, and findings were extrapolated to cefazolin.1 Another study done in 1976 showed low cefazolin concentrations in CSF after only a single administration of a 1-gram dose intravenously.2
However, studies on cefazolin and CSF concentrations were not abandoned completely. A few years later, another study found the concentration of cefazolin in CSF after one administration of a 2-gram dose was actually higher than the concentration of one administration of a 2-gram dose of nafcillin, a drug we commonly rely on to treat meningitis. Since then, multiple other studies supported this idea that the concentration of cefazolin in the CSF may be higher and more effective than previously reported. Importantly, the studies indicated that concentrations of cefazolin reaching the CSF are above the recommended breakpoints for Staphylococcus aureus and Enterobacterales.4 Further data is needed to ensure optimal dosing, but some experts recommend high doses of 2 grams administered intravenously every 6 hours or a continuous infusion of 8 to 10 grams daily.
Fact: Overall, cefazolin administered at higher doses may be considered as an option for CNS infection treatment with proper susceptibilities.
- Mangi RJ, Kundargi RS, Quintiliani R, Andriole VT. Development of meningitis during cephalothin therapy. Ann Intern Med 1973; 78:347–51.
- Bassaris HP, Quintiliani R, Maderazo EG, Tilton RC, Nightingale CH. Pharmacokinetics and penetration characteristics of cefazolin into human spinal fluid. Curr Ther Res Clin Exp 1976; 19:110–20.
- Frame PT, Watanakunakorn C, McLaurin RL, et al. Penetration of nafcillin, methicillin, and cefazolin into human brain tissue. Neurosurgery 1983;12:142–7.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 31st ed. CLSI document M100. Wayne, PA:CLSI, 2021.
Myth 2: Linezolid should be avoided in patients taking Selective Serotonin Reuptake Inhibitors
Linezolid is an antibiotic commonly used for multidrug-resistant gram-positive bacterial infections. Linezolid is a nonselective inhibitor of monoamine oxidase which can reduce the breakdown of serotonin. This process leads to a potential increased risk of serotonin syndrome (SS) in patients taking other serotonergic drugs.1
One study of over 4,000 patients taking serotonergic agents found a low incidence of SS overall and no significant increase in frequency in patients taking linezolid.2 This was further supported by additional studies evaluating individual antidepressants and linezolid combinations. The scatterplot below shows the relationship between the proportion of SS reports (on the x-axis) and the mean number of DDIs (on the y-axis) for each serotonergic agent.3
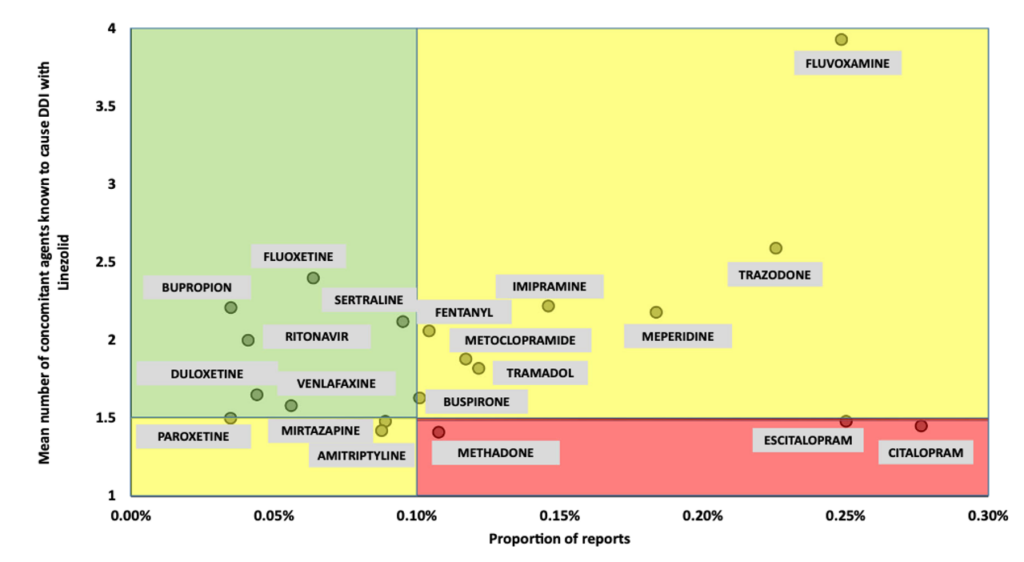
Fact: Overall, serotonin syndrome is exceedingly rare even when linezolid is combined with serotonergic agents. If linezolid is needed as the preferred antibiotic, the risk/benefit profile in patients receiving serotonergic drugs is acceptable.
1. US FDA drug safety communication: updated in-formation about the drug interaction between linezolid (Zyvox) and serotonergic psychiatric medications. Available at: www.fda.gov/Drugs/DrugSafety/ucm276251.htm. Accessed 25 September 2022.
2. Butterfield JM, Lawrence KR, Reisman A, et al. Comparison of serotonin toxicity with concomitant use of either linezolid or comparators and serotonergic agents: an analysis of phase III and IV randomized clinical trial data. J Antimicrob Chemother 2012; 67:494–502.
3. Gatti M, Raschi E, De Ponti F. Serotonin syndrome by drug interactions with linezolid: clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2021;77(2):233-239. doi:10.1007/s00228-020-02990-1
Myth 3: Linezolid dosing regimens do not need renal dose adjustment
According to the package insert for linezolid, the dosing regimen is 600 mg twice daily administered IV or orally with no indicated adjustment for renal dysfunction. The initial clinical trials and uses of linezolid in short courses had low incidences of thrombocytopenia at <3%. However, a study in 2019 suggested a higher risk of thrombocytopenia at 27% incidence with a 13.8% incidence of severe thrombocytopenia. Interestingly, it was found that an increased linezolid exposure was associated with thrombocytopenia. The frequency of thrombocytopenia was higher in patients with a renal dysfunction (eGFR <60 ml/min/1.73m) at 42.9% compared to patients without renal dysfunction at 16.8%.1 This information leads to an expert recommendation of a reduced dose of linezolid for renal dysfunction so as to reduce the incidence of thrombocytopenia.
Fact: The current expert recommendation is to reduce the linezolid dose to 300 mg administered IV or orally twice daily in patients with an eGFR <60 ml/min/1.73m.2
1. Crass RL, Cojutti PG, Pai MP, Pea F. Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob Agents Chemother. 2019;63(8):e00605-19. doi:10.1128/AAC.00605-19
2. Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med 2020; 46:1127–53.
Myth 4: Clindamycin is recommended as a first-line treatment for surgical site infection prophylaxis in patients with penicillin allergies
Cefazolin is typically the first-line choice for surgical prophylaxis but is often avoided in patients with a penicillin allergy due to historical references that quote potential cross-reactivity of 5-10%.1 In these cases, clindamycin has been a common alternative choice for patients with penicillin allergies since it does not share any structural similarities with beta-lactam antibiotics. However, the 5-10% cross-reactivity with cefazolin is likely overstated, as the side chain of cefazolin is not similar to any other beta-lactam, including other cephalosporins. A meta-analysis found that incidence of allergy to both penicillin and cefazolin was 0.7%.2 Importantly, a 2023 study indicated a lower frequency of surgical site infections when cefazolin was used prophylactically compared to clindamycin.3
In 2022, Nebraska Medicine implemented a systemwide change that suppressed alerts for non-IgE-mediated penicillin allergies in the electronic medical record upon cephalosporin prescribing. In individuals with penicillin allergy, preoperative cefazolin prescribing increased from 49.6% to 74.3% (P < .01).5
Fact: The Joint Task Force on Practice Parameters recommends cefazolin as a first-line antibiotic for surgical prophylaxis in patients with penicillin allergies.4
- Herbert ME, Brewster GS, Lanctot-Herbert M. Medical myth: ten percent of patients who are allergic to penicillin will have serious reactions if exposed to cephalosporins. West J Med 2000; 172:341
- Sousa-Pinto B, Blumenthal KG, Courtney L, et al. Assessment of the frequency of dual allergy to penicillins and cefazolin: a systematic review and meta-analysis. JAMA Surg 2021; 156:e210021.
- Norvell MR, Porter M, Ricco MH, et al. Cefazolin vs. second-line antibiotics for surgical site infection prevention after total joint arthroplasty among patients with a beta-lactam allergy [manuscript published online ahead of print 24 April 2023]. Open Forum Infect Dis 2023. doi:10.1093/ofid/ofad224
- Khan DA, Banerji A, Blumenthal KG, et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol 2022; 150:1333–93.
- Bogus, A., McGinnis, K., May, S., Stohs, E., Schooneveld, T., & Bergman, S. Perioperative cefazolin prescribing rates following suppression of alerts for non-IgE-mediated penicillin allergies. Antimicrobial Stewardship & Healthcare Epidemiology, 3(S2), S98-S99. doi:10.1017/ash.2023.369