Microbe Monday is a monthly installment featuring a microbe of clinical or scientific importance. This month, in recognition of STI Awareness month, we discuss Chlamydia trachomatis. The following content was provided by Natalie Sturd and Dr. Elizabeth Rucks– experts in Chlamydial biology at UNMC.
See here for our previous Microbe Monday posts.
Chlamydia trachomatis causes the most common bacterial sexually transmitted disease in both developed and developing nations, and rates of C. trachomatis infections have been steadily rising since the CDC began data collection in 19841. In developing countries, it is also the causative agent of blinding trachoma, which is the leading cause of preventable blindness. In the United States, we spend almost $700 million in direct medical costs towards treating chlamydial infections, which is second only to what we spend treating HIV and HPV. While there are readily available and effective antibiotic treatments for chlamydial infections, the challenges we face in health care involve the asymptomatic nature of most infections, as well as the pathologic consequences of repeat and/or chronic infections.
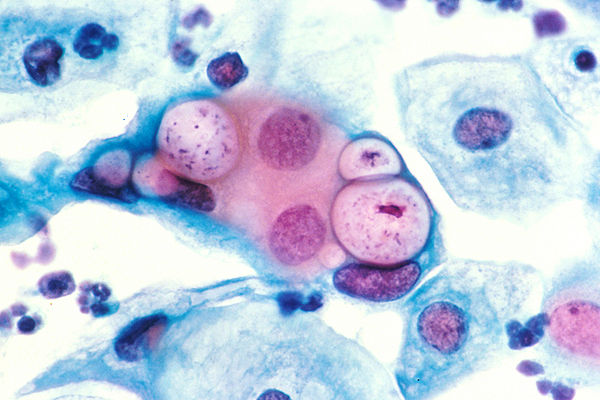
Indeed, for genital infections, 70-80% of infections in women and 40-50% in men are asymptomatic2, which means these infections regularly go unnoticed and untreated. Both untreated and repeat infections increase the risk of developing more serious chronic sequelae1. In the female reproductive tract, this can present as pelvic inflammatory disease (PID) and fibrosis of the reproductive tract, leading to ectopic pregnancy and tubal infertility. Current treatment regimens for chlamydial infections include the use of broad-spectrum antibiotics like doxycycline and/or azithromycin3, which can lead to the development of bacterial vaginosis (BV)4,5. BV is marked by an imbalance of vaginal microbiota with an increase of Lactobacillus and/or Garderella species and is treated with metronidazole, which indiscriminately eliminates both undesirable and desirable members of the vaginal microbiome3. Thus, despite awareness and available treatment, chlamydial infections remain a significant source of patient morbidity and constitute a serious financial burden on our healthcare system.
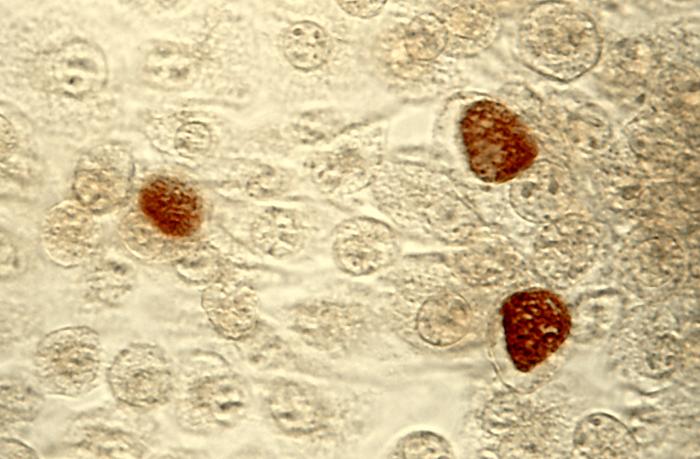
During the 6 million years that C. trachomatis has evolved with its human host6, its genome underwent reductive evolution, meaning it lost many of the genes found in other prokaryotes and retained a much smaller genome (~1.04Mbp; ~895 open reading frames). As a result of genome reduction, C. trachomatis is an obligate intracellular pathogen, meaning it requires the host cell to complete its developmental cycle. Chlamydia has a distinct biphasic developmental cycle, alternating between two morphological forms: the infectious, non-replicative elementary body (EB) and the non-infectious, replicative reticulate body (RB). The developmental cycle begins with EB entry into the host cell, where it establishes an intracellular niche within a pathogen-specific vacuole, termed the inclusion. The EB rapidly differentiates into the RB, which continues to divide within the inclusion. During later stages of the developmental cycle, RBs asynchronously undergo secondary differentiation to create new EBs. Importantly, each stage of chlamydial development is marked by distinct patterns of gene transcription, as each stage has different nutritional, proteomic, and molecular requirements to continue to grow and avoid detection by the host. From within the inclusion, C. trachomatis must mediate specific host interactions and these interactions are critical for chlamydial pathogenesis. A family of chlamydial effector proteins known as inclusion membrane proteins (Incs) are the primary mediators of these kinds of interactions and are a primary focus in our lab. Inc-host protein interactions have been implicated in the modulation of cellular survival pathways, vesicular trafficking of exocytic vesicles, and inhibition of the host innate immune response, all of which are hypothesized to contribute to a successful infection and a limited host response.
Watch for our next post this week where we will feature the Rucks lab and dive into the research exploring this important pathogen conducted at UNMC.

We in the Rucks lab hope to contribute towards improving prevention and infection outcomes from a basic science approach. Specifically, our work helps 1) understand how C. trachomatis establishes an infection in human tissues and 2) identify the outcome of infection in the host cells/tissues in regard to cell biology.
References
1. Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2020. Atlanta: U.S. Department of Health and Human Services; 2022.
2. Shetty S, Kouskouti C, Schoen U, et al. Diagnosis of Chlamydia trachomatis genital infections in the era of genomic medicine. Brazilian Journal of Microbiology. 2021;52(3):1327-1339.
3. Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187.
4. Tamarelle J, Ma B, Gajer P, et al. Nonoptimal Vaginal Microbiota After Azithromycin Treatment for Chlamydia trachomatis Infection. J Infect Dis. 2020;221(4):627-635.
5. Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17(8):e235-e279.
6. Nunes A, Gomes JP. Evolution, phylogeny, and molecular epidemiology of Chlamydia. Infect Genet Evol. 2014;23:49-64.