Recently, a multidisciplinary team at UNMC published a diagnostic stewardship study in Infection Control & Hospital Epidemiology (ICHE) entitled: Hardwiring diagnostic stewardship using electronic ordering restrictions for gastrointestinal pathogen testing, that prompted a press release from The Society for Healthcare Epidemiology of America (SHEA), and a feature on an upcoming ICHE podcast episode. Drs. Jasmine Marcelin and Trevor Van Schooneveld are the lead and senior authors on this study.
What is the study about?
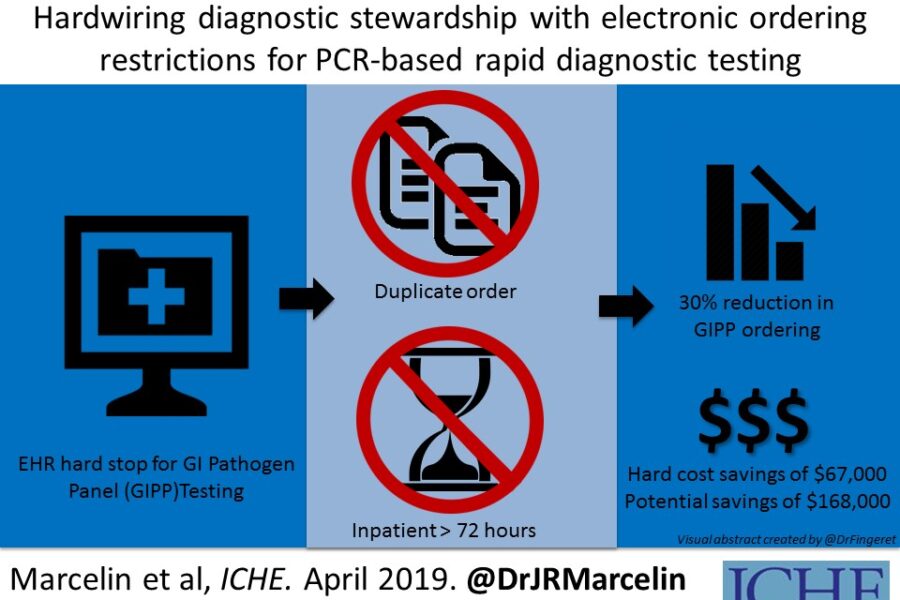
Antimicrobial stewardship has really morphed from just being about antibiotic prescribing, to a more comprehensive strategy including diagnostic testing and collaboration with the microbiology laboratory. Our antimicrobial stewardship program at University of Nebraska Medical Center is heavily invested in diagnostic stewardship and the gastrointestinal pathogen panel (GIPP) was the focus of this latest project. The hospital implemented the use of the GI Pathogen panel in 2016, a quick and sensitive, but costly test that detects 22 common organisms causing diarrheal illness. Many studies using this test have found it useful to detect a wide range of community-associated organisms, but that it is not as helpful if patients have been hospitalized for more than 3 days, or have had a previously negative test. After implementing the test (replacing most traditional stool cultures) in 2016, it was used frequently and repeatedly, including in patients hospitalized even longer than 5 days.
 There is a clinical effectiveness team at UNMC (including physicians, lab clinicians, pharmacists, nurses and other leaders) at the hospital that looks at things like this to see where we can be more effective at providing high-value, cost conscious care to our hospitalized patients. The GI pathogen panel test is not cheap, and it was being used frequently, so this was a good target for the CE team to tackle. Collaborating with the microbiology lab, we used our hospital electronic health record (EHR) to provide best practice alerts when people tried to order the test, and if someone tried to order it inappropriately (which we designated as >72hrs post admission or duplicate test), there was a hard stop that prevented the test from being signed and completed. If people felt very strongly that the test needed to be done after the hard stop, they could call the microbiology laboratory director to discuss overriding the stop (that did not occur very often).
There is a clinical effectiveness team at UNMC (including physicians, lab clinicians, pharmacists, nurses and other leaders) at the hospital that looks at things like this to see where we can be more effective at providing high-value, cost conscious care to our hospitalized patients. The GI pathogen panel test is not cheap, and it was being used frequently, so this was a good target for the CE team to tackle. Collaborating with the microbiology lab, we used our hospital electronic health record (EHR) to provide best practice alerts when people tried to order the test, and if someone tried to order it inappropriately (which we designated as >72hrs post admission or duplicate test), there was a hard stop that prevented the test from being signed and completed. If people felt very strongly that the test needed to be done after the hard stop, they could call the microbiology laboratory director to discuss overriding the stop (that did not occur very often).
What did the study find?
In the fifteen months before the hard stop was activated, we found that 21.5 percent of the GIPP tests ordered were inappropriate. In the 15 months after implementing the hard stop in the EHR, only 4.9 percent were inappropriate (P<0.001). The study also included a cost analysis for this intervention. When considering only the orders prevented by the hard stop (30% reduction), there was an actual savings of $67,000 over that post-intervention 15 months. However, if considering all of the potential orders which were prevented by the best practice alerts AND the actual hard stop preventions, there was a 46% reduction in inappropriate testing and potential savings of $168,000, even after accounting for the cost of alternative testing! That is enough to fully fund an ID pharmacist or at least partially fund an ID physician for a hospital’s Antimicrobial Stewardship team.
Why is this study interesting?
The study will be useful for any clinicians who may order microbiologic testing for diarrheal illness for hospitalized patients – primary and specialist physicians, microbiologists and laboratory directors, medical trainees, and advanced practice providers. Additionally, hospital administrators and quality improvement individuals will be interested in this study given the significant cost savings associated with this intervention.  When this work was presented at IDWeek2018, many people were looking for simple interventions like this that saved hospitals money, as examples to demonstrate to their C-suites the value of funding Antimicrobial Stewardship. This study is interesting because it shows a relatively simple intervention can have an impactful result. It demonstrates that that when it comes to diarrheal illnesses in the hospital, asking physicians to reconsider if the testing is appropriate through hardwired alerts saves money without compromising quality of care.
When this work was presented at IDWeek2018, many people were looking for simple interventions like this that saved hospitals money, as examples to demonstrate to their C-suites the value of funding Antimicrobial Stewardship. This study is interesting because it shows a relatively simple intervention can have an impactful result. It demonstrates that that when it comes to diarrheal illnesses in the hospital, asking physicians to reconsider if the testing is appropriate through hardwired alerts saves money without compromising quality of care.
What about future research questions?
The most important takeaway from this study is that we can improve the efficiency of the care we deliver by hardwiring criteria for appropriate test ordering and diagnostic stewardship into the electronic health record, and that it is something relatively easy to do. Some things that were not studied, but would have been helpful if we collected information on include: length of stay or antibiotic use for diarrheal illness. This would have had allowed the opportunity to evaluate the potential relationship of these outcome metrics with the hard stop. That would not only further support our assertion that the hard stop would not compromise quality of care, but if we could demonstrate shorter length of stay or fewer unnecessary antibiotic prescriptions for this indication, we could go even further to state that this intervention enhances quality of care.  Another area of interest would be applying this concept to other rapid diagnostic testing. Currently, other available multiplex tests like the GI pathogen panel include the meningitis panel, upper respiratory and newer lower respiratory panel tests; if we can figure out how to use these tests responsibly and not abuse them, we could further improve high-value, high-efficiency and cost-conscious care.
Another area of interest would be applying this concept to other rapid diagnostic testing. Currently, other available multiplex tests like the GI pathogen panel include the meningitis panel, upper respiratory and newer lower respiratory panel tests; if we can figure out how to use these tests responsibly and not abuse them, we could further improve high-value, high-efficiency and cost-conscious care.
Citation
Marcelin, J., Brewer, C., Beachy, M., Lyden, E., Winterboer, T., Murphy, C., . . . Van Schooneveld, T. (2019). Hardwiring diagnostic stewardship using electronic ordering restrictions for gastrointestinal pathogen testing. Infection Control & Hospital Epidemiology, 40(6), 668-673. doi:10.1017/ice.2019.78