Pharm to Exam Table: Clinical Pharmacology/Antimicrobial Updates – Trogarzo® (ibalizumab-uiyk), a new monoclonal antibody treatment approved for multi-drug resistant HIV-1
On March 6th 2018, the Food and Drug Administration approved a new monoclonal antibody called ibalizumab-uiyk, marketed under the tradename Trogarzo®. Ibalizumab-uiyk (IBA) is an intravenous treatment administered every two weeks. It is intended for use in combination with other antiretrovirals (in an optimized background regimen), for the treatment of HIV-1 infection in heavily treatment-experienced adults with multidrug resistant HIV-1 infection, failing their current antiretroviral regimen.(1)
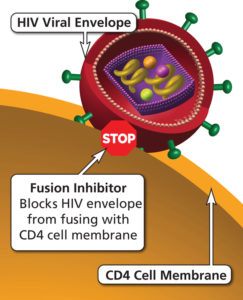 IBA is a humanized long-acting IgG4 monoclonal antibody that prevents attachment of the HIV-1 molecule to CD4+ T cells by changing the conformation of the CD4+ T cell receptor while preserving the function of the cell. These functions classify IBA as an entry inhibitor.(1,3,4) Other previous entry inhibitors are enfurvitide (fusion inhibitor) and maraviroc (CCR5 antagonist).
IBA is a humanized long-acting IgG4 monoclonal antibody that prevents attachment of the HIV-1 molecule to CD4+ T cells by changing the conformation of the CD4+ T cell receptor while preserving the function of the cell. These functions classify IBA as an entry inhibitor.(1,3,4) Other previous entry inhibitors are enfurvitide (fusion inhibitor) and maraviroc (CCR5 antagonist). 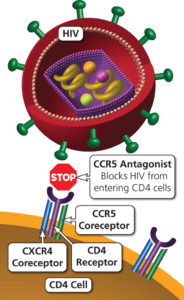
The FDA approval was based on results of study TMB-301 (N=40), an open-label study that investigated the antiviral activity and safety of IBA when administered with an optimized background regimen (OBR) in treatment-experienced patients with multi-drug resistant HIV-1. The study followed patients prospectively over 24 weeks after dosing them with IBA and their OBR. At day 14, 83% of patients achieved at least a 0.5 log10 (roughly 70%) viral load reduction from baseline seven days after receiving the IBA loading dose. By week 25, the mean change from baseline viral load was -1.6 log10 with 55% of participants having a ≥1 log10 reduction in viral load. The most common drug-related adverse reactions (incidence ≥ 5%) were diarrhea (8%), dizziness (8%), nausea (5%) and rash (5%). Serious adverse events were reported in 23% (9/40) of participants and of these only one was considered drug-related (immune reconstitution inflammatory syndrome, IRIS).(1,2,4)
IBA is the first monoclonal antibody entry inhibitor approved for HIV infection. Given its unique properties, tolerable safety profile, lack of drug-drug interactions or antiretroviral cross-resistance, IBA offers an attractive antiretroviral option for adjunct treatment of multi-drug resistant HIV-1 infection in combination with an Optimized Background Regimen.
References
1 Trogarzo ® [package insert]. TaiMed Biologics USA Corp., Irvine, California; 2018
2 TaiMed Biologics Inc. Ibalizumab Plus Optimized Background Regimen in Patient With Multi-Drug Resistant HIV. Available from: https://clinicaltrials.gov/ct2/show/NCT02475629
3 Iacob S A, Iacob D G. Ibalizumab targeting CD4 receptors, an emerging molecule in HIV therapy[J]. Frontiers in microbiology, 2017, 8: 2323.
4 Lewis S et al. Long-acting ibalizumab in patients with multi-drug resistant hiv-1: a 24-week study. CROI, 2017 (Poster).
Images courtesy the Department of Health and Human Services AidsInfo website HIV/AIDS glossary on CCR5 Antagonists and Fusion Inhibitors.
Thanks to Chao Fu, UNMC PharmD candidate 2018 for providing concept for content.